MOVR: Neuromuscular Observational Research
MOVR's Legacy: About Us
The Neuromuscular Observational Research (MOVR) Data Hub was created as part of MDA’s commitment to empowering individuals living with neuromuscular diseases to live longer, more independent lives. Launched over a decade ago, MOVR addressed a critical data gap in the neuromuscular space by pioneering new strategies to accelerate data collection and accessibility for researchers, clinicians, and drug developers.
One of its core strategies was to leverage the strength of the MDA Care Center Network. By capturing data across this national system, MOVR provided valuable insights into disease progression, informed regulatory submissions, and served as a rich source of real-world evidence for post-approval processes.
Disease Indications Included
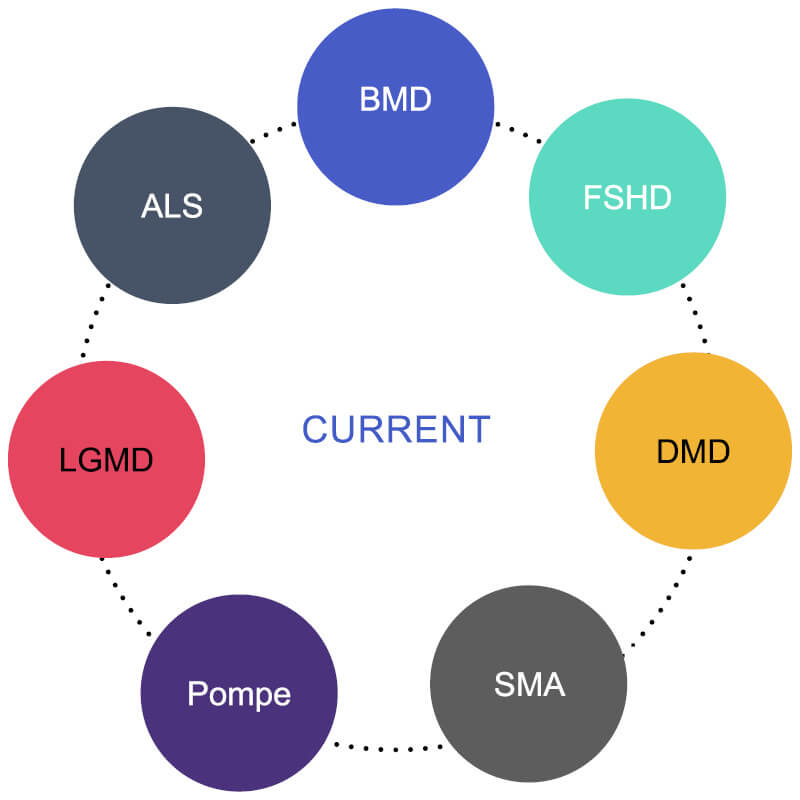
MOVR focused on seven neuromuscular diseases with high research activity and clearly defined care standards:
- ALS, BMD, DMD, FSHD, LGMD, Pompe disease, and SMA These conditions were selected because they had multiple investigational therapies in development, standardized data elements identified by national working groups, and established standards of care. This alignment allowed MOVR to uniquely measure care adherence and its impact on outcomes.
Data Collection Framework
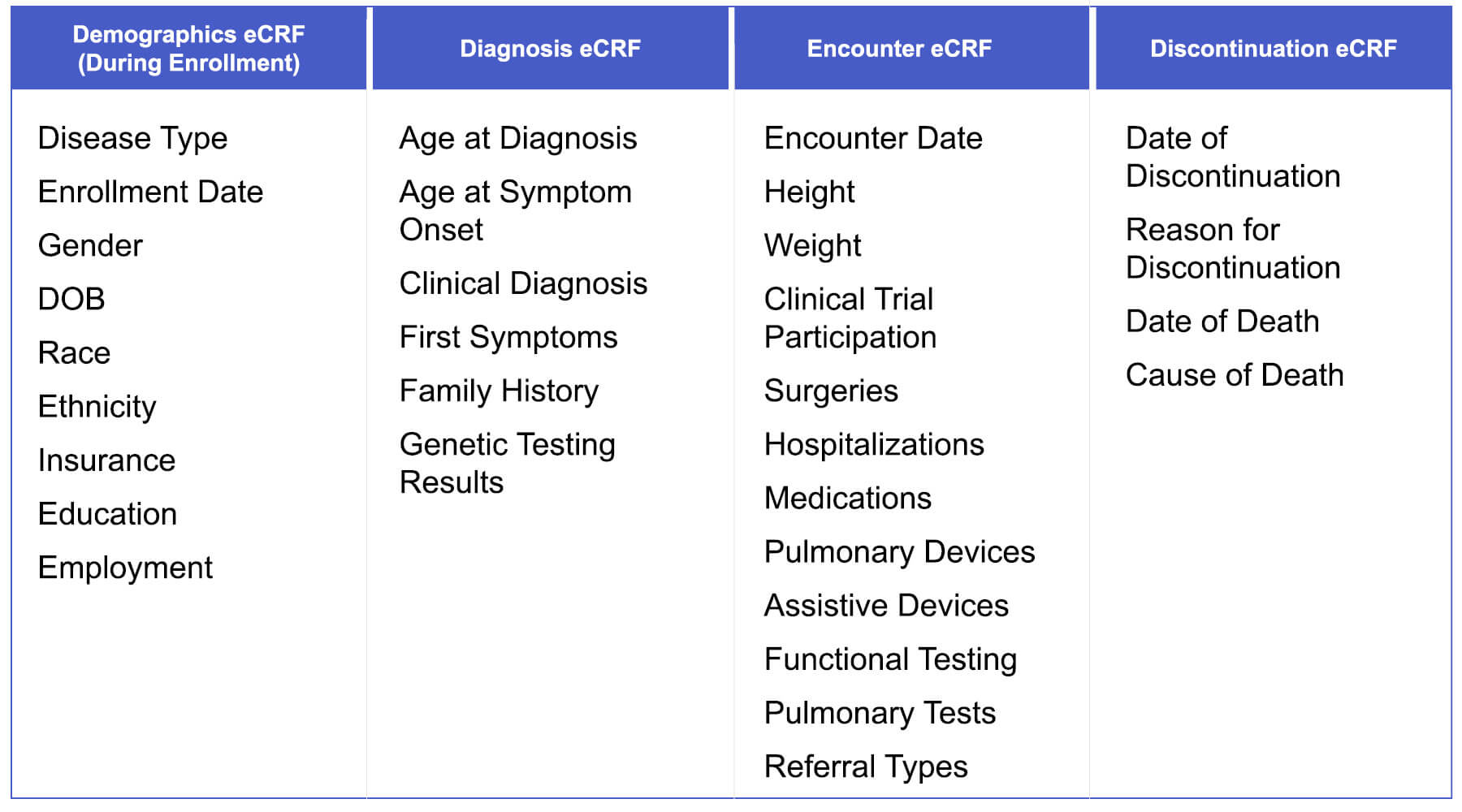
MOVR captured 31 core data elements using four electronic case report forms (eCRFs):
- Demographics
- Diagnosis
- Encounter
- Discontinuation
Each indication also included additional, condition-specific data elements. Clinical research staff at participating MDA Care Centers entered data directly from the patients’ electronic health records.
Why MOVR Was Created
Rapid advancements in diagnostics and treatment options were reshaping the landscape of neuromuscular medicine. As emerging therapies shifted from supportive care to disease-modifying interventions, MOVR was designed to help overcome barriers to widespread access and implementation.
By aggregating anonymized clinical and genetic data from patients receiving care across the Care Center Network, MOVR enabled researchers to better understand therapeutic outcomes, improve trial design, and explore how these diseases affect individuals differently. It also empowered clinicians to identify patients who might benefit from new treatments or clinical research opportunities. Thirty-one core data elements are captured across four electronic case report forms (eCRFs) – Demographics eCRF, Diagnosis eCRF, Encounter eCRF, and Discontinuation eCRF. Additional unique data elements are captured for each indication. Data are entered into the eCRFs from the electronic health record by clinical research staff at participating MDA Care Centers.
MOVR Publications, Presentations and Real-World Guidance
- The Muscular Dystrophy Association's neuroMuscular ObserVational Research Data Hub (MOVR): Design, Methods, and Initial Observations
- MDA's MOVR Data Hub Captures Longitudinal Clinical Data Across 7 Neuromuscular Diseases to Evaluate Current Care Practices and the Impact of FDA Approved Therapies
- MDA's MOVR Data Hub provides insights into adoption of approved therapies for neuromuscular disease
- Evaluating MDA's MOVR Data Hub as a Source for Real-World Data
MOVR 2.0 – The Next Chapter in Neuromuscular Research
Building on the foundation of the original MOVR Data Hub, the Muscular Dystrophy Association (MDA) is now launching the next evolution of its national patient data platform: MOVR 2.0.
What Is MOVR 2.0?
MOVR 2.0 is a secure, research-driven platform designed with—and for—individuals living with neuromuscular disease. Unlike traditional data tools, MOVR 2.0 centers the patient experience, prioritizing the health data that truly matters to the community. By sharing personal medical histories, participants will drive innovation in treatment development, help researchers better understand disease impact, and improve clinical care delivery.
Focused Beginnings
The early testing phase of MOVR 2.0 began with a six-month pilot program focused exclusively on individuals living with spinal muscular atrophy (SMA). This thoughtful rollout ensures the platform meets real-world needs, functions seamlessly, and reflects the voice of the community from the outset.
A Future Fueled by Collaboration
MOVR 2.0 represents a major step toward building an inclusive, scalable platform that accelerates research and improves outcomes across the entire neuromuscular disease landscape. By uniting scientific progress with patient partnership, it sets the stage for a more connected and empowered future in neuromuscular care.
Quick Links:
- MDA Medical Education
- Grants at a Glance
- Research Grants
- Creating a New Therapy
- MDA Venture Philanthropy
- MOVR: Neuromuscular Observational Research
- Newborn Screening for Neuromuscular Diseases
- Cost of Illness of Neuromuscular Diseases in the US
- Contact Our Research Team
- MDA Kickstart Program
- Telemedicine Resources