Science That Changes Lives
From breakthroughs in the lab to real-world progress—accelerating research that delivers results for families today.
Grants at a Glance
MDA’s research program awards grants to the world’s best scientists investigating promising theories and therapies that may accelerate treatments and cures for families living with muscular dystrophy, ALS and related neuromuscular diseases.
Grant – Winter 2011 – DMD - Bradley Olwin, Ph.D.
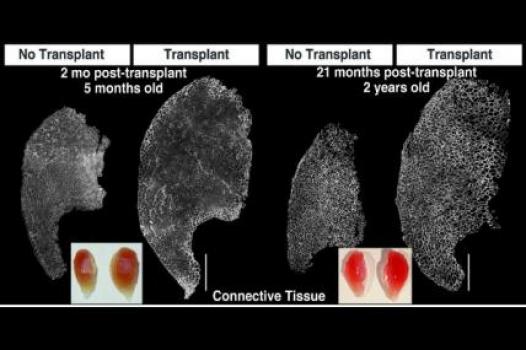
MDA has awarded a research grant totaling $369,165 over three years to Bradley Olwin, professor of molecular, cellular & developmental biology at the University of Colorado in Boulder. The new funds will help support Olwin’s study of muscle regeneration in injured and diseased skeletal muscle — particularly in the muscular dystrophies, including Duchenne muscular dystrophy (DMD).
Olwin is a longtime MDA grantee, having received funding from the Association almost continuously since the early 1980s.
Diseases such as the muscular dystrophies that result in progressive loss of skeletal muscle tissue reflect disruption of the normal muscle regenerative processes. If the cells responsible for normal repair could be augmented by drug therapies or cell replacement therapies, regeneration might be enhanced, slowing or halting the loss of skeletal muscle function.
In previous studies, Olwin and colleagues identified a rare stem cell that they hypothesize is a primary source of skeletal muscle stem cells (also called "satellite" cells). The team plans to study the newly identified cells and uncover any contributions they may make to the regeneration process in injured and diseased skeletal muscle. Next, the team will transplant the cells into the muscles of a research mouse with a DMD-like disease in order to determine how well they restore muscle function, slow muscle scarring (called fibrosis), and produce the protein missing in DMD, dystrophin.
Findings from Olwin’s studies may result in new drug-based or cell-based strategies to enhance new muscle growth in progressive muscle diseases.
"I have been funded by the MDA for the majority of my career as a research scientist, beginning in the early 1980s," Olwin said. "Without MDA funding, I may not have continued in skeletal muscle research."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Bradley Olwin, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - DMD — Diego Fraidenraich, Ph.D.

Diego Fraidenraich, an assistant professor in the department of cell biology & molecular medicine at the University of Medicine and Dentistry of New Jersey, was awarded an MDA grant totaling $375,000 over three years. The funds will support his study of the relationship between muscle and fat formation in a mouse model of Duchenne muscular dystrophy (DMD).
These mice, like humans with DMD, lack the dystrophin protein in their skeletal muscles and heart because of a mutation in the dystrophin gene. They develop a muscle disease resembling human DMD.
Recently published reports have shown that skeletal muscle and fat have a common cellular ancestor, and Fraidenraich's team intends to understand the similarities and differences in the development of these two tissues.
In previous experiments, Fraidenraich and colleagues treated DMD mice with stem cells from healthy mice, so that the dystrophin protein was present in some, but not all, of their cells.
These so-called "mosaic" mice developed some normal muscle tissue from the stem cells, as well as some fat tissue that had muscle-like characteristics. The researchers termed this unusual, stem-cell-derived tissue "muscularized fat."
In his new work, Fraidenraich will seek to understand more about this muscle-like fat derived from the transplanted stem cells and define its possible role in the development of muscle. He'll also try to increase the ratio of muscle to fat in the DMD mice.
Fraidenraich hopes to provide new understanding of muscle formation that will supply leads for future therapies for DMD and perhaps other forms of muscular dystrophy.
He has received MDA funding since 2007, and says, "Continuous funding from MDA has allowed me to develop and expand my research program. The support has been instrumental to my research and my lab."
Funding for this MDA grant began August 1, 2011.
Grantee: DMD — Diego Fraidenraich, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Wilfried Rossoll

MDA awarded $358,653 to Wilfried Rossoll, assistant professor at Emory University in Atlanta, for research into the effects on nerve cells, or "motor neurons," of toxic TDP43 protein, implicated in ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
"Recently, we have developed novel tools to study protein localization, trafficking and function in cultured primary motor neurons," Rossoll said. "Funding from MDA will now allow us to apply these methods to address the biological role of the ALS disease protein TDP43 in motor neurons."
TDP43 protein is present in all body tissues and serves multiple roles in the processing of messenger RNAs (mRNAs), the molecules that carry genetic information from DNA to the sites of protein synthesis. However, the function of TDP43 in motor neurons and vulnerability of those cells to the protein still is not understood.
"Most studies on the role of TDP43 protein have used non-neuronal cell lines, so its function in the highly specialized motor neuron cell type remains unclear," Rossoll said. "We will use cell cultures of primary motor neurons and motor neurons generated from stem cells to study the function of TDP43 in this cell type, and to gain information about its involvement in ALS."
Preliminary data generated by Rossoll's team suggests that TDP43 plays a role in mRNA processing in the long axon fibers that conduct electrical impulses to muscle cells. The team will test the hypothesis that decreased levels of TDP43 in mRNA-protein complexes in these axons may contribute to the degeneration of motor neurons in ALS.
Increased understanding of the molecular and cellular functions of TDP43 could help lead to the development of therapies that delay or even prevent motor neuron degeneration in people with ALS or other neurodegenerative diseases.
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Wilfried Rossoll
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Stanley H. Appel

MDA awarded a research grant totaling $330,000 to Stanley H. Appel, chair of the department of neurology at the Methodist Neurological Institute (MNI) in Houston, to study the protective effects of a specific class of immune system cells in ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Appel, a longtime MDA adviser and director of the MDA/ALS center at MNI, will look at T cells, observed in people with ALS as well as in mouse models of the disease, and known to be involved in protecting motor neurons (nerve cells that activate muscles).
Appel's lab previously has documented that T cells can protect neurons in a mouse model of ALS, at least in part by enhancing the neuroprotective functions of another type of immune system cell called microglia. When activated, these cells can produce helpful proteins, but they also can cause dangerous inflammation.
Finding ways to protect motor neurons is a key goal in the development of ALS therapeutics, and Appel's group is working to understand the interaction among other cells in the immune system responsible for prompting T cells to protect neurons.
Project plans include the transplantation of various T-cell types into ALS research mouse models in order to determine which of the cells are most helpful to neurons. A greater understanding of this T-cell population and its associated molecular signals may lead to therapeutics based on increasing the numbers and effectiveness of these cells to help protect neurons in people with ALS.
"MDA has been a critical source of support throughout the development of our immunological studies in ALS," Appel said. "It's permitted us to assemble a team of scientists focused on the neuroinflammatory processes in ALS, and to substantiate the importance of protective immunomodulation in ALS."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Stanley H. Appel
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Shanthini Sockanathan
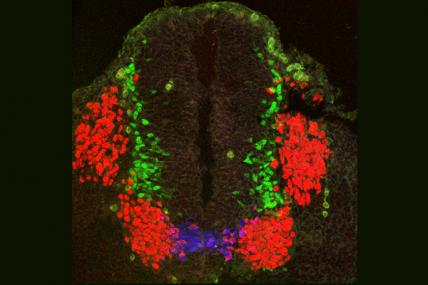
MDA awarded a grant totaling $347,832 to Shanthini Sockanathan, associate professor of neuroscience at the Johns Hopkins University School of Medicine in Baltimore, for research into the molecular causes of nerve cell, or motor neuron, degeneration in diseases including ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Sockanathan and colleagues will study the Gde2 mouse, a new model of motor neuron degeneration that they developed based on studies of the maturation, or "differentiation," of motor neurons in embryonic mouse spinal cords.
The Gde2 mouse lacks a protein called GDE2. Mouse embryos lacking the protein possess significantly decreased numbers of motor neurons, but at birth they contain numbers of these cells equivalent to those of control mice. As adults, the Gde2 mice experience a significant loss of motor neurons, with the remaining nerve cells indicating progressive degeneration.
In their new work, the investigators will study the physical and biological characteristics of Gde2 mice, and monitor the progression of motor neuron degeneration in older animals. Because the GDE2 protein normally is active in both differentiating and fully matured motor neurons, the team plans to remove it in mice at different times during development to determine at which stage or stages GDE2 loss causes degeneration.
"MDA's clear commitment to funding basic research projects has been instrumental in my ability to develop and maintain an active research program dedicated to understanding the mechanisms of neuronal differentiation," Sockanathan said. "Indeed, my first grant as an independent investigator was from MDA, and this funding helped me establish my research program and, importantly, provided me with the flexibility to chart a new direction in my work. Over the years, I have been fortunate to receive more funding from MDA, and in each case this funding has helped me broaden my research program in novel areas."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Shanthini Sockanathan
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – CNM/MTM - Jocelyn Laporte, Ph.D.
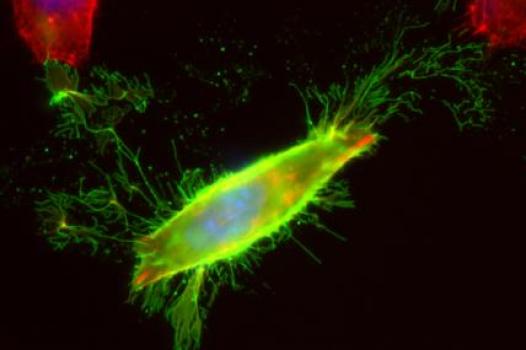
MDA has awarded a research grant totaling $341,250 over three years to Jocelyn Laporte at the University of Strasbourg, France. The new funds will help support Laporte’s efforts to identify genes responsible for centronuclear/myotubular myopathies (CNM)/(MTM).
Laporte noted that his lab identified some of the first genes associated with these types of diseases in 1996, but that a number of other CNM-causing genes remain to be identified. Using a sophisticated technology called "high-throughput sequencing," also known as "massively parallel sequencing," Laporte and colleagues plan to uncover new genes associated with CNM.
The main challenge of this novel approach is the identification of the causative mutation among the many DNA variants that are not connected to the myopathy. It will require special effort to analyze the data.
Findings from Laporte’s work likely will facilitate diagnosis of CNM in individuals, improve genetic counseling services, and lend insight to better care and follow-up after diagnosis. In addition, the identification of causative genes will help inform scientists of the underlying disease mechanisms, help researchers determine eligibility for human clinical testing, and provide new targets at which to aim potential new therapies.
"MDA funding is a key factor for successful research on rare muscle diseases," Laporte said.
Funding for this MDA grant began February 1, 2011.
Grantee: CNM/MTM - Jocelyn Laporte, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - CMD — Sonja Nowotschin, Ph.D.
Sonja Nowotschin, a postdoctoral research fellow in the developmental biology department at the Sloan-Kettering Institute in New York, has been awarded an MDA development grant totaling $163,638 over three years. (Development grants are MDA's mechanism for furthering the career development of promising young researchers.)
The funding will help support Nowotschin's research on how muscles are formed at the embryo stage, with the goal of understanding and ultimately treating congenital muscular dystrophies (CMDs) and congenital myopathies, such as myotonia congenita, paramyotonia congenita,central core disease (CCD), nemaline myopathy,myotubular/centronuclear myopathies (MTM/CNMs) and periodic paralysis.
Nowotschin and her colleagues will study embryonic mice to investigate the origin and development of the skeletal muscles. They'll identify and study the stem cells that form muscles in mice with and without genetic mutations.
"Knowledge gained from these studies will assist logical efforts to design new therapeutic strategies," Nowotschin said.
"Importantly," she added, "by investigating the molecular mechanisms operating in the embryo, we will be able to formulate the molecular principles that should assist in developing methods to reprogram adult, or 'differentiated,' cells." Cells that have matured (differentiated) into muscle tissue and have then been reprogrammed back into stem cells are being considered as possible therapies to treat muscle disease.
MDA funding, Nowotschin said, "is essential to move this project forward."
Funding for this MDA grant began August 1, 2011.
Grantee: CMD — Sonja Nowotschin, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Oliver Hobert
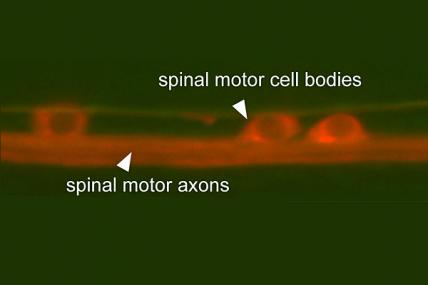
MDA awarded $374,511 to Oliver Hobert, professor of biochemistry and molecular biophysics at Columbia University, New York, to study the function of the TDP43 gene, mutations in which can cause ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
"In order to diagnose and treat ALS, it is important to understand the molecular events that underlie this disease," Hobert said. The TDP43 gene, known to cause ALS in humans, "works in a manner that is not understood. Our goal is to better understand the function of this gene."
Using the invertebrate C. elegans (nematode, or roundworm) model, Hobert's team will study the TDP43 gene in order to determine its function and possible interaction with other genes.
"Funding by the MDA means a great deal to us — not just because of the financial support but also because it motivates us to direct our basic research toward questions that are medically relevant," Hobert said.
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Oliver Hobert
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Michael Benatar
Michael Benatar, associate professor of neurology and epidemiology at Emory University in Atlanta, received an MDA grant totaling $525,000 to continue research into the early stage of FALS — familial, or inherited, ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease) — prior to symptom onset.
With funding from MDA, Benatar began his "pre-FALS" study in 2007, tracking a group of 30 people at risk for developing ALS because they harbor a mutation in the SOD1 gene, known to cause some forms of the disease. His team collected a series of physical, functional and neurological data over time in an effort to discern early biological markers ("biomarkers") of the disease process, and established a repository of biological samples collected from the study participants.
In Benatar's new follow-up study, the original group of 30 participants will expand to include presymptomatic individuals with mutations in other ALS susceptibility genes such as TDP43 and FUS.
Study aims include better definition of the presymptomatic stage of familial ALS, identification of environmental factors that might modify the age of disease onset among genetically susceptible individuals, continued development of biomarkers of early disease, and expansion of the existing collection of biological specimens.
Benatar's exploration into the presymptomatic phase of ALS could facilitate earlier recognition and diagnosis of both the familial and sporadic forms of the disease, which in turn could point the way toward therapeutics aimed at prevention or delay of ALS onset, as well as attempts to stop or slow the disease before it causes irreversible damage.
"MDA has shown great foresight in recognizing the importance of this study and the kind of long-term contribution that it can make to our understanding of the disease," Benatar said.
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Michael Benatar
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – CMT - Thien Nguyen, M.D., Ph.D.

MDA has awarded a research grant totaling $420,000 over three years to Thien Nguyen, assistant professor in the department of neurology at Johns Hopkins University School of Medicine in Baltimore. The new funds will help support Nguyen’s research into the breakdown of peripheral nerves (nervous tissue that connects the spinal cord with muscles and sensory organs) in Charcot-Marie-Tooth disease (CMT).
Degeneration of the axons (the long fibers that carry signals from nerve cell bodies to muscle) is a hallmark of type 1 CMT and is the primary determinant of the severity of symptoms in people with the disease. It follows that prevention of axonal degeneration should improve clinical outcomes for people with CMT1.
The same principle also should be applied to other types of CMT, Nguyen noted.
In previous studies, Nguyen and colleagues have determined that mutations in genes for myelin-making cells called "Schwann cells" can lead to mutant Schwann cells that may instruct axons to degenerate through certain specific signaling molecules that normally promote stability and survival, including one called myelin-associated glycoprotein (MAG). Study results out of Nguyen's lab have suggested that another signaling molecule, called netrin-1, may play an important role in axonal survival in CMT, making it a potential therapeutic target.
The investigators will determine whether increasing netrin-1 activity might prevent axonal degeneration in cell culture and in a research mouse model.
Findings from Nguyen’s work could reveal the potential therapeutic benefit of netrin-1 and lead to its development as a neuroprotective therapeutic for CMT.
"In theory, results from the project also could be expanded to axonal degeneration even without demyelination," Nguyen said. "Thus, there could be applicability to many of the neurodegenerative disorders covered by MDA."
Funding for this MDA grant began February 1, 2011.
Grantee: CMT - Thien Nguyen, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – CMD - Shireen Lamande, Ph.D.
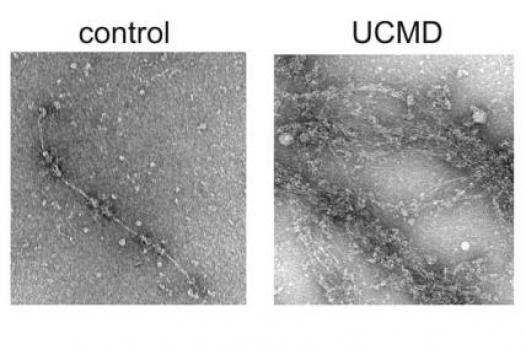
MDA has awarded a research grant totaling $251,596 over two years to Shireen Lamande, senior research fellow and group leader for muscular dystrophy research and musculoskeletal disorders at Murdoch Childrens Research Institute in Parkville, Victoria, Australia. The new funds will help support Lamande’s research into the identification of new genes responsible for two types of congenital muscular dystrophy (CMD), Bethlem myopathy and Ullrich congenital muscular dystrophy.
Lamande and colleagues plan to identify new genes responsible for collagen 6-related congenital muscular dystrophies, and determine how and why mutations associated with the genes cause muscle disease.
To date, the study team has screened 100 people with Bethlem myopathy and Ullrich CMD, and has found collagen 6 mutations in 62. The remaining 38 do not have collagen 6 mutations, an indication that other, as yet unidentified, genes also underlie these disorders.
Of the 38 individuals whose mutations were unknown, the investigators have identified new mutations in 11. They now plan to use a variety of methods to examine the newly uncovered genes, and identify those that are new muscular dystrophy candidate genes.
Results from Lamande’s work should improve the diagnostic process for individuals and families, and increase scientists’ understanding of muscle biology and disease.
Funding for this MDA grant began February 1, 2011.
Grantee: CMD - Shireen Lamande, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Jean-Pierre Julien

MDA awarded a grant totaling $345,000 to Jean-Pierre Julien, professor at Laval University, Canada, for research into genetic variations in a protein called chromogranin B (CHGB) that has been shown to modify disease risk and hasten onset in a type of familial ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
In previous studies, Julien's group discovered that CHGB interacts with mutant, or flawed, forms of the superoxide dismutase 1 (SOD1) protein associated with SOD1-mediated familial, or inherited, ALS.
The team studied variations in the chromogranin B gene, which carries the instructions for the CHGB protein, in a large group of people with and without ALS. Results showed that people with ALS were twice as likely to have a common CHGB variant called P413L than those without the disease. The P413L CHGB variant also correlated with a 2.2-fold greater relative risk for development of ALS, and among those with the disease, onset of symptoms occurred an average 10 years earlier for those with the variant than for those without it.
In its new work, Julien's group will conduct experiments in cultured nerve cells and in a strain of mice engineered to carry the human CHGB variant to test the hypothesis that P413L increases vulnerability of motor neurons. The investigators also aim to determine whether the variant affects CHGB's interaction with mutant SOD1 and uncover the exact mechanism by which it increases risk of ALS and modifies disease onset.
"MDA funding is timely and needed," Julien said, "to determine how this particular genetic variant increases risk of ALS and hastens disease onset."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Jean-Pierre Julien
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – CMD - Madeleine Durbeej-Hjalt, Ph.D.
MDA has awarded a research grant totaling $442,023 over three years to Madeleine Durbeej-Hjalt, a professor in muscle biology at Lund University (Sweden), Department of Experimental Medical Science. The grant will help support Durbeej-Hjalt's study of muscle-protein degradation processes in type 1A congenital muscular dystrophy (CMD1A).
CMD1A is caused by mutations in the gene for laminin alpha 2, a protein strand in a larger protein called merosin that connects muscle fibers to their surrounding tissue. Without the laminin alpha 2 strand, the normally three-stranded merosin protein can't perform this connective function and severe neuromuscular dysfunction results.
Although evidence indicates that loss of laminin alpha 2 leads to CMD1A, the underlying molecular mechanisms that cause muscle damage and loss in the disease remain unclear. Durbeej-Hjalt and colleagues hypothesize that muscle degeneration may be caused, at least in part, by increased destruction of muscle proteins through one or both of two major cellular protein degradation systems: the cellular "garbage can" known as the proteasome, or via the autophagic-lysosomal pathway, in which cell components are degraded and recycled through the cellular "recycle bin," or lysosome.
Durbeej-Hjalt's team has data indicating that activity in both protein degradation systems is increased when laminin alpha 2 is missing, and the investigators plan to test whether treatment with proteasome or autophagy inhibitors can block protein degradation and lead to improvement in muscle health.
Findings from Durbeej-Hjalt’s new study may generate important preclinical data that could lead to the development of therapies for people with CMD1A.
"I am extremely grateful for the MDA funding I have received since 2005," Durbeej-Hjalt said. "Without the MDA grants, my research group would not exist today."
Funding for this MDA grant began February 1, 2011.
Grantee: CMD - Madeleine Durbeej-Hjalt, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Dena Jacob

Research scientist Dena Jacob at Thomas Jefferson University in Philadelphia, received an MDA grant totaling $180,000 for research into decreasing cells' resistance to therapeutic medications in ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease).
Jacob plans to study a "transporter" protein called P-glycoprotein, or P-gp, which belongs to a class of "transporter" proteins that pump drugs out of cells. P-gp recognizes a broad range of drugs and normally is found in cells of the blood brain and blood spinal cord barriers and also in some affected brain tissue, limiting drug penetration.
"P-gp expression in the spinal cord itself may impart overt pharmacoresistance (drug resistance) to ALS-affected cells and account for the poor therapeutic effect observed in [a number of previous] ALS clinical trials," Jacob said.
Jacob's findings could lead to re-examination of many previously unsuccessful clinical drug trials and encourage the development of new, combined therapeutic strategies for ALS.
"I am extremely honored and excited to be a Development Grant recipient of the MDA, an organization whose efforts and support are essential for advancing our understanding of neuromuscular diseases," Jacob said. "This funding will enable me to complete important experiments that will help us identify improved therapeutic strategies to target the mouse model of ALS and, ultimately, humans with this disease."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Dena Jacob
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Daniela Zarnescu
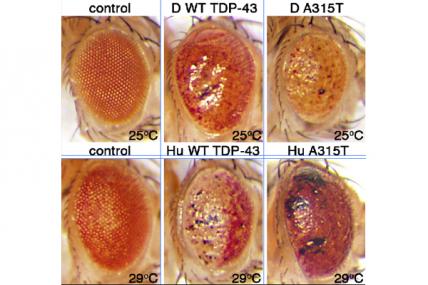
MDA awarded $375,000 to Daniela Zarnescu, assistant professor in neuroscience at the University of Arizona in Tucson, Ariz., to conduct gene and drug discovery research in a drosophila fruit fly model that carries a mutation in the TDP43 gene associated with a genetic form of human ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Alterations in TDP43 gene activity in the fruit fly model lead to symptoms that mimic those found in humans with the disease, including the death of nerve cells called motor neurons and the formation of abnormal clumps of cellular material, called inclusions, that contain TDP43 proteins.
Zarnescu's group will conduct genetic screening tests on the flies in order to identify other genes that interact with TDP43, some of which may be involved in disease causation or progression. The group also will screen drugs in search of one or more that can rescue the defects in the fruit fly model.
The work could lead to novel therapeutic targets and approaches for ALS.
"Recognizing the need to fund such projects is not only an important step to ensure important discoveries in the field, but it also shows that MDA is at the forefront of the effort towards identifying new therapies and a potential cure for ALS," Zarnescu said.
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Daniela Zarnescu
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Daniel Offen

Daniel Offen, head of the neurology laboratory at Tel-Aviv University, Israel, received an MDA grant totaling $359,700 for research into a combined cell and gene therapy approach for ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Offen's research team will engineer progenitor cells, a type of immature cell that forms muscle, to express various combinations of neurotrophic factors (proteins that support motor neuron health and which previously have been reported to be beneficial in rodent models of ALS). The investigators will inject mixtures of progenitor cells expressing different combinations of the neurotrophic factors into muscles in the SOD1 research mouse model of ALS and then monitor the course of the disease.
Behavioral, physical and biochemical survival indicators will be observed in order to analyze the effects of the stable activity of different growth factors on disease progression, extension of life span and quality of life in ALS. The experiments with the most promising results will then be duplicated using immature human muscle cells.
It's hoped the work will lead to a new cell and gene therapy strategy for treatment of human ALS.
"Past MDA support has enabled us to develop several approaches to isolate and propagate myogenic cells in cultures, some of which will be used in the present investigation," Offen said. "Our current MDA award will enable us to conduct the proposed project and hopefully to contribute to the treatment of ALS patients."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Daniel Offen
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - ALS — Brian Freibaum

MDA awarded $180,000 to research scientist Brian Freibaum at St. Jude Children's Research Hospital in Memphis, Tenn., for research into the mechanism by which toxic TDP43 protein leads to the development and progression of some forms of ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease).
Prior research has suggested that mutant (flawed) TDP43 protein is a critical component in the development of some forms of ALS, although the mechanism by which it works remains unknown. Freibaum has planned a three-tiered approach to uncover the protein's role in the disease.
First, Freibaum's team will test for toxicity in various tissues of fruit flies engineered to carry the human TDP43 gene. Using different mutant (flawed) and shortened forms of the protein is expected to help pinpoint what parts of the TDP43 protein are required for disease progression.
Next, the team will use human cell lines and mouse motor neurons (nerve cells that control muscle) to explore how TDP43 interacts with other proteins, where it normally localizes in the cell, and whether these locations are altered with mutant forms of the protein. Further experiments will be used to determine what other proteins interact with normal and flawed TDP43 protein.
Finally, the group will use the fruit fly model to perform genetic screens designed to explore how other proteins cooperate with mutant TDP43 to induce toxicity in motor neurons.
Greater understanding of the role TDP43 plays in ALS will help provide a more targeted approach to the development of new therapies.
"I am honored to be funded by such a prestigious organization that has a strong history in the advancement of neuromuscular disease research," Freibaum said. "This funding will be of great significance to our lab’s ALS research."
Funding for this MDA grant began August 1, 2010.
Grantee: ALS — Brian Freibaum
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2013 - SMA — Umrao Monani, Ph.D.
Umrao Monani, associate professor at Columbia University Medical Center in New York City, was awarded an MDA research grant totaling $300,000 over a period of three years to study how junctions between neurons and muscle are affected in spinal muscular atrophy (SMA).
SMA is due to a gene mutation that causes a deficiency in a protein calledSMN. Without it, the nerve cells that control muscles, called motor neurons, die off and muscles weaken. One of the first effects of lack of SMN is seen at the neuromuscular synapse, the place on the surface of the muscle where the motor neuron contacts it and sends its signal to control muscle contraction.
“We are interested in investigating the role of SMN in the formation, function, maintenance and repair of these structures,” says Monani. “We will use model mice, in which defects of the neuromuscular synapses become apparent when the SMN protein is depleted, to determine how SMN might shape synaptic structure and function.
"By examining molecular changes that occur normally in the motor neurons and muscle as the synapses mature, and by looking for differences when SMN is reduced, we hope to identify specific mediators of the SMA disease process. Understanding how low SMN levels translate into a disease that affects the motor system is not only of enormous scientific interest but is also likely to teach us how best to effectively treat SMA and other diseases of the motor neurons,” he says.
Funding for this MDA grant began August 1, 2013.
Grantee: SMA — Umrao Monani, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - SMA — Bennett Novitch, Ph.D.
Bennett Novitch, assistant professor of neurobiology at the University of California, Los Angeles, was awarded an MDA research grant totaling $300,000 over a period of three years to study the development of motor neurons that control respiration and their significance for spinal muscular atrophy (SMA).
SMA is due to the loss of motor neurons (nerve cells that control muscle activity), and in the most severe forms of SMA, the motor neurons controlling respiration are affected early in the disease.
“These observations raise the possibility that these respiratory defects might have a developmental origin [that is, they may arise in the developing embryo or fetus],” Novitch says, “resulting either from the improper formation of respiratory motor neurons, or their integration into functional motor circuits. This issue has been difficult to address, however, as our knowledge of the developmental origins of respiratory motor neurons and their assembly into circuits is incomplete.”
In this study, Novitch will study development of respiratory motor neurons and their organization into motor circuits that carry out both inspiration and expiration (breathing in and out). He also will study the gene networks that control this development. Better understanding of these networks, and the developmental processes they control, may lead to better understanding of how respiratory motor neurons are affected in SMA.
“Our study will provide new insights into the root causes of SMA and potentially lead to the discovery of new therapeutic targets. Moreover, by studying the process by which respiratory motor circuits are initially formed, we will gain vital information on how this activity may be recapitulated to rebuild damaged circuits to help patients maintain their ability to breathe independently,” he says.
Funding for this MDA grant began August 1, 2013.
Grantee: SMA — Bennett Novitch, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2013 - SBMA/ALS — Constanza Cortes, Ph.D.

Constanza Cortes, a postdoctoral researcher at the University of California, San Diego, was awarded an MDA development grant totaling $177,410 over a period of three years to study the role of a cell recycling system inspinal-bulbar muscular atrophy (SBMA or Kennedy disease) andamyotrophic lateral sclerosis (ALS).
Both SBMA and ALS are characterized by the death of motor neurons, the nerve cells that control movement. Their causes are different, but in both diseases, there are defects in autophagy, a process cells use to break down and recycle large proteins and other cellular substances. Autophagy is critical for cell health, but relatively little is known about the process in neurons, Cortes says. She will be using both light microscopy and electron microscopy to examine how the function of one regulator of the process — a protein called TFEB — may be altered by interaction with the mutant protein that causes SBMA.
Working with cell culture, mouse models and cells from patients, Cortes says, “We expect to develop autophagy-intervention therapeutics and test these approaches in our mice, to determine the feasibility of manipulating autophagy as a therapeutic strategy for neuromuscular diseases. In this project, I hope to demonstrate the importance of neuronal autophagy for neuromuscular disease, and advance the field in ways that will benefit patients suffering from these debilitating disorders.”
Funding for this MDA grant began August 1, 2013.
Grantee: SBMA/ALS — Constanza Cortes, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant – Winter 2011 – ALS/SMA - Michael Miller, Ph.D.
MDA has awarded a research grant totaling $350,133 over three years to Michael Miller, associate professor in the department of biology at the University of Alabama, Birmingham. The grant will help support Miller's research into the fundamental molecular mechanisms responsible for the death of nerve cells called motor neurons in ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease) and spinal muscular atrophy (SMA).
Using nematode (worm) and fly research models, Miller and colleagues have generated preliminary data suggesting that mutations in genes associated with familial (inherited) ALS and SMA disrupt a signaling mechanism that controls function of the cellular "energy factories" called mitochondria, which are necessary for motor neuron health.
Now, using a nematode model, the study team plans to combine biochemical and genetic manipulation with the ability to directly monitor changes in the worm’s cellular mitochondria as a means of uncovering the fundamental molecular mechanisms responsible for motor neuron death in ALS and SMA — mechanisms the team hypothesizes may influence mitochondrial function.
Miller expects the major impacts of his work will be the identification of mitochondria as the linchpin in neurodegenerative disorders including ALS and SMA, and the long list of drug targets his team expects to identify through its screening process.
"Funding by the MDA for this project is critically important," Miller said, "as without this support, the momentum gained over the past few years would come to a halt."
Funding for this MDA grant began February 1, 2011.
Grantee: ALS/SMA - Michael Miller, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2013 - SBMA — Albert La Spada, M.D., Ph.D.
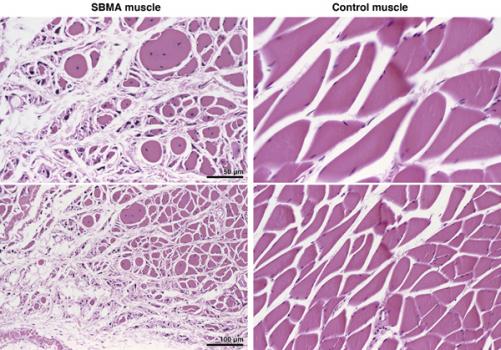
Albert La Spada, professor of cellular and molecular medicine, neurosciences and pediatrics at the University of California, San Diego, was awarded an MDA research grant totaling $300,000 over a period of three years to study the causes of neurodegeneration (loss of nerve cells) inspinal-bulbar muscular atrophy (SBMA).
SBMA, also known as Kennedy disease, is an adult-onset neuromuscular disorder affecting men. It is caused by a mutation in the gene for theandrogen receptor protein, and it leads to the death of motor neurons, which are needed to control muscle contraction.
La Spada has created a mouse model and neuronal cell culture models of SBMA. He will now use these models to investigate how the SBMA-causing mutation leads to the death of motor neurons, focusing on changes in metabolism and impaired degradation of worn-out or defective proteins.
La Spada’s lab will test drugs that target a proposed regulatory factor and also will see whether blocking the erroneous androgen receptor gene instructions with compounds known as antisense oligonucleotides is beneficial. In previous work funded by MDA, La Spada’s lab has shown that directing therapies to muscle cells, rather than directly to the nerve cells that die in SBMA, can prevent the disease in mice.
“This exciting and unexpected finding represents an important step toward successful therapy development, as treatments that target muscle [instead of nerve cells] should be more straightforward to create, easier to test in patients and less likely to cause serious side effects,” La Spada says. “This key finding also suggests that a similar strategy is worth considering for related motor neuron diseases, such as spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS).”
Funding for this MDA grant began August 1, 2013.
Grantee: SBMA — Albert La Spada, M.D., Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant – Winter 2011 – ALS - Shanthini Sockanathan, Ph.D.
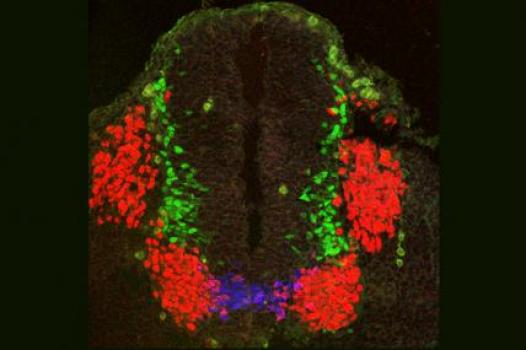
MDA has awarded a research grant totaling $396,000 over three years to Shanthini Sockanathan, associate professor of neuroscience at Johns Hopkins School of Medicine in Baltimore. The new funds will help support Sockanathan’s study of motor neuron (nerve cell) development in neurodegenerative diseases such as ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease).
In previous work, Sockanathan and colleagues showed that a protein called GDE2 controls the production of spinal motor neurons through extracellular glycerophosphodiester phosphodiesterase (GDPD) activity.
More recently, the team discovered that GDE2 activity is itself regulated by a second protein, Prdx1. Prdx1 activates GDE2 by breaking a particular coupling called a "disulfide bond" inside GDE2 that normally inhibits GDE2 function. Thus, the disulfide bond within GDE2 acts a molecular switch that turns motor neuron production "on" or "off." By extension, the pathways that modify disulfide bond formation play pivotal roles in the control of motor neuron differentiation (maturation).
One question that emerges from this work is: How are active forms of Prdx1 generated to ensure efficient initiation of GDE2-dependent motor neuron differentiation?
Sockanathan's study team will test the hypothesis that inactive forms of Prdx1 are reactivated through separate pathways.
In addition, building off their recent finding of another Prdx molecule that is expressed with GDE2 and inhibits its activity, the team suggests the possibility that this mechanism regulates the initiation, extent and rate of motor neuron differentiation. The team will test this theory using a combination of different approaches.
Taken together, these studies are expected to provide insight into how the pathways that modify disulfide-bond formation intersect to control the onset and progression of motor neuron differentiation.
Funding for this MDA grant began February 1, 2011.
Grantee: ALS - Shanthini Sockanathan, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – ALS - Jiou Wang, M.D., Ph.D.
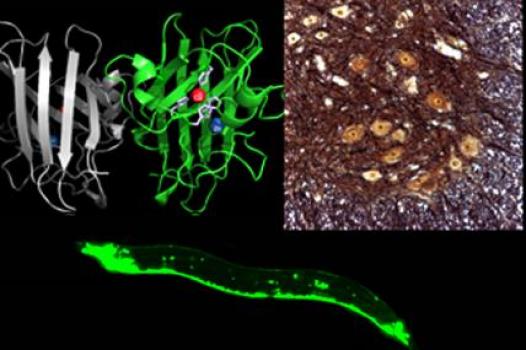
MDA has awarded a research grant totaling $330,000 over three years to Jiou Wang, assistant professor of biochemistry & molecular biology and neuroscience at Johns Hopkins University in Baltimore. The new funds will help support Wang’s study of the molecular mechanisms underlying ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease).
"Genetic and protein discoveries in humans have opened the door to studying the disease in genetically-engineered animal models," Wang said. "The nematode, with its short lifespan and well-conserved nervous system, along with the availability of powerful genetic tools, provides a unique opportunity for dissecting the disease mechanisms of ALS."
With colleagues, Wang previously has established a nematode model of ALS that carries a mutation in the SOD1 gene (associated with the familial form of human ALS). In his new work, Wang and his study team plan to use the nematode model to compare the molecular mechanisms of toxicity in both the SOD1 protein and in another protein associated with ALS, TDP43. They aim to uncover the mechanisms by which the TDP43 and SOD1 disease genes trigger neurotoxicity, and then determine whether the same holds true in other mammals. It’s hoped in-depth analysis of these mechanisms will reveal pathways at which researchers can target effective therapeutics.
"Support from MDA is very special to us, as it allows us to continue our research into ALS," Wang said. "Since my graduate studies, I have focused my research on understanding the basic mechanisms of this devastating disease. Now, as an early-stage independent investigator, MDA support is critical in allowing me to continue to work on ALS."
Funding for this MDA grant began February 1, 2011.
Grantee: ALS - Jiou Wang, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – ALS - James Berry, M.D.
MDA has awarded a clinical research training grant totaling $180,000 to clinical research fellow James Berry at Massachusetts General Hospital (MGH) in Boston. The grant will support completion of a two-year fellowship during which Berry plans to study the effects of a drug called ISIS-333611 in familial, or inherited, ALS (amyotrophic lateral sclerosis, or Lou Gehrig’s disease).
Familial ALS accounts for 5 to 10 percent of all ALS cases. About 20 percent of those (1 to 2 percent of all ALS cases) are caused by a mutation in the SOD1 gene, which leads to production of abnormal SOD1 protein.
Berry is a member of the study team currently planning a phase 2 clinical trial of intrathecal (into the spinal canal) administration of the experimental drug ISIS-333611 (made by Isis Pharmaceuticals of Carlsbad, Calif.) in people with familial ALS. The trial will be a follow-on study to a phase 1 trial of the drug, currently under way, if results from that trial are favorable.
Previous studies of the drug in rats have shown that it is safe and tolerable, that it reduces mutant SOD1 RNA (the chemical step between DNA and protein synthesis) and protein levels, and slowed progression of the disease. It's thought that decreasing mutant SOD1 levels in humans may confer similar therapeutic benefits.
The planned phase 2 study will test ISIS-333611 over an extended period of time to assess its safety and tolerability. If the phase 2 trial is successful, a phase 3 trial may be planned to test efficacy of the drug.
"MDA funding is critical to my career development and my ability to begin my career as a clinical researcher in ALS," Berry said. "I am incredibly excited to have received this award, and I am looking forward to getting started on the project."
Funding for this MDA grant began February 1, 2011.
Grantee: ALS - James Berry, M.D.
Grant type: Clinical Research Training Grant
Award total:
Institution:
Country:
Learn more about the research projects MDA is currently funding:
- MDA Awards 25 Grants Totaling More Than $6.6 Million for Neuromuscular Disease Research
- Muscular Dystrophy Association Awards 26 Grants Totaling More Than $7.5 Million for Neuromuscular Disease Research
Research Across Diseases
As part of MDA's basic research program, the grants we fund focus on advancing basic science and generating ideas for potential drug therapies through projects initiated by the researchers themselves. Through the projects they fund we will learn more about the processes that drive neuromuscular diseases. We’ll identify, validate and optimize biological targets at which to aim future therapies. We’ll test potential therapeutic strategies, develop drug development tools and make other advances that will help pave the way to more clinical trials.
Twice a year, grant applications are reviewed by MDA’s Research Advisory Committee which recommends the best projects for approval. Funding is approved by MDA’s Board of Directors.
- MDA Medical Education
- Grants at a Glance
- Research Grants
- Creating a New Therapy
- MDA Venture Philanthropy
- MOVR: Neuromuscular Observational Research
- Newborn Screening for Neuromuscular Diseases
- Cost of Illness of Neuromuscular Diseases in the US
- Contact Our Research Team
- MDA Kickstart Program
- Telemedicine Resources
Find MDA
in your Community
-

Grants at a Glance
Read More -

Search for Clinical Trials
Learn More