Search our Grants
MDA’s research program awards grants to the world’s best scientists investigating promising theories and therapies that may accelerate treatments and cures for families living with muscular dystrophy, ALS and related neuromuscular diseases.
Grant - Summer 2011 - FSHD — Michael Kyba, Ph.D.

MDA awarded a research grant totaling $375,000 over three years to Michael Kyba, assistant professor in the Lillehei Heart Institute and department of pediatrics at the University of Minnesota in Minneapolis. The funds will help support Kyba's efforts to identify and test experimental therapies in facioscapulohumeral muscular dystrophy (FSH, or FSHD).
The mutation associated with FSHD is a contracted (deleted) segment of DNA in a region of chromosome 4 called D4Z4. The D4Z4 region consists of a series of repeated DNA sequences. It is not yet fully understood how the contraction causes the disease, but scientists have found that it appears to modify the packaging of this part of chromosome 4 so that a gene named DUX4, which is encoded within each D4Z4 repeat, becomes active.
Kyba and colleagues are developing mice that carry the DUX4 gene. The team aims to identify and test drug- and genetic-based inhibitors of DUX4 in the mice, the most promising of which potentially may lead to experimental therapies for FSHD.
"This research project is directed at developing a treatment for FSHD," Kyba said. "As MDA is dedicated to curing muscular dystrophy, this is a natural partnership. Support from MDA is critical to our success."
Funding for this MDA grant began August 1, 2011.
Grantee: FSHD — Michael Kyba, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant – Winter 2011 – SM - Claudio Sette, Ph.D.
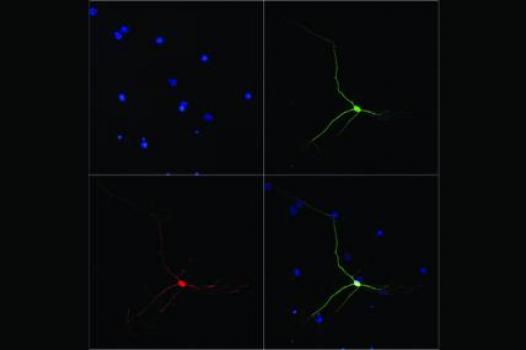
MDA has awarded a research grant totaling $219,000 over three years to Claudio Sette, associate professor for the department of public health and cell biology at the University of Rome Tor Vergata in Rome, Italy. The new funds will help support Sette’s study of the molecular mechanisms underlying spinal muscular atrophy (SMA).
SMA is caused by mutations in the SMN1 gene, which leads to a deficiency of the protein SMN (for "survival of motor neuron") and subsequent degeneration and death of the motor neurons in the spinal cord. Although SMN2, a gene almost identical to SMN1, is present in humans, it's unable to compensate for SMN1's loss due to differences in the two genes' protein-building instructions. With the exception of the case in which an occasional error may result in a functional or partially functional protein, SMN2-derived proteins are short, highly unstable, and for the most part nonfunctional.
Sette and colleagues intend to test a variety of ways to modulate the SMN2 gene, forcing it to mimic the SMN1 gene and process its protein-building instructions in such a way as to lead to the production of functional SMN protein and prevention of motor neuron degeneration and death. Results from Sette’s work should increase understanding of the molecular mechanisms responsible for SMA — in particular, the molecular defects responsible for motor neuron degeneration. In addition, the group’s studies may lead to new therapeutic approaches for treatment of the disease.
Funding for this MDA grant began February 1, 2011.
Grantee: SM - Claudio Sette, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – SBMA - J. Paul Taylor, M.D., Ph.D.

J. Paul Taylor, associate member of St. Jude Children’s Research Hospital in Memphis, Tenn., has received an MDA research grant totaling $330,000 over three years. The funds will help support Taylor’s continued research into a number of possible therapeutic targets in spinal-bulbar muscular atrophy (SBMA).
In prior research funded by MDA, Taylor and colleagues developed a fruit fly model of SBMA and used it to determine how mutations in the androgen receptor (AR) gene lead to the death of motor neurons (nerve cells) and deterioration of muscle in this disease.
Specifically, the study team determined that toxicity occurs only when mutant AR enters the cell nucleus and binds to DNA. The team determined that toxicity is mediated by a small interaction surface on AR called "AF2." The team also has determined that toxicity is strongly enhanced by a chemical modification called "sumoylation."
Now, Taylor intends to continue along the same line of study, testing the validity of two therapeutic targets, AF2 and sumoylation, identified in his previous work.
The team will engineer new mouse models of SBMA, some carrying normal forms of AR and others carrying mutant forms of the protein that are defective in DNA binding, incapable of undergoing sumoylation, or have a disrupted or nonfunctional AF2 surface. In parallel, the team will work to identify small molecule inhibitors of these targets in their fruit fly model.
Findings derived from Taylor’s studies could lead to the identification of compounds that can be developed for human clinical testing.
"Funding from MDA has been essential to my research program," Taylor said. "Our previous findings were made possible by MDA, and this new grant from MDA is essential to translating those findings into meaningful therapies."
Funding for this MDA grant began February 1, 2011.
Grantee: SBMA - J. Paul Taylor, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – Mito. Myopathy - Antoni Barrientos, Ph.D.

MDA has awarded a research grant totaling $346,500 over three years to Antoni Barrientos, an associate professor in the departments of neurology and biochemistry and molecular biology at the University of Miami (Florida) Miller School of Medicine. The grant will help support Barrientos' study of the underlying molecular mechanisms in some forms of mitochondrial myopathy.
In the mitochondrial myopathies, commonly caused by the deficiency of an enzyme called cytochrome c oxidase (COX), cellular energy production is severely compromised, affecting brain, muscle and other organs with high energy demands. A complete understanding of COX synthesis is essential for uncovering the molecular basis underlying these diseases.
Barrientos and colleagues recently identified in a yeast research model two "chaperone" proteins responsible for helping assemble the COX enzyme.
Now, Barrientos plans to study the human equivalents of the two yeast proteins to determine whether their functions in humans are comparable. To do this, his team will use a gene silencing technique to "turn off" the genes that carry instructions for production of the chaperones, essentially inhibiting the proteins' production and enabling the investigators to identify what happens (or what doesn't happen) in their absence.
Barrientos' previous MDA-funded work has resulted in significant contributions to scientists' understanding of factors involved in COX assembly and regulation of the COX biogenetic process. Now, findings from his new work potentially may lead to therapeutics based on targeting the genes that carry instructions for the two COX assembly chaperones.
"MDA support is extremely important to our laboratory," Barrientos said, adding, "I have been working on cytochrome c oxidase assembly for the last 12 years, beginning with my postdoctoral studies at Columbia University, always with the support of MDA."
Funding for this MDA grant began February 1, 2011.
Grantee: Mito. Myopathy - Antoni Barrientos, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MTM - James Dowling, M.D., Ph.D.
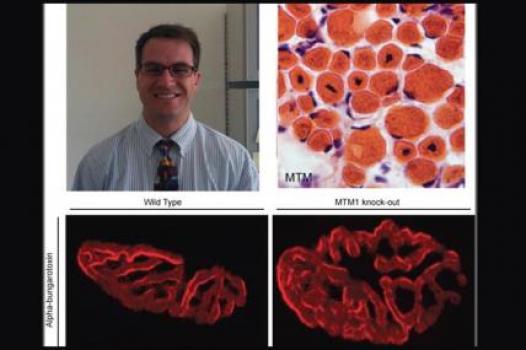
MDA has awarded a research grant totaling $353,679 to James Dowling, assistant professor in the departments of pediatrics and neurology at the University of Michigan Medical Center in Ann Arbor. The funds will help support Dowling's research into potential therapies for myotubular myopathy (MTM).
For his latest line of research, Dowling has partnered with Christopher Pierson, an assistant professor in the department of neuropathology at Nationwide Children's Hospital in Columbus, Ohio.
Recently the two identified abnormalities in a part of the muscle called the neuromuscular junction (NMJ), the meeting point of nerve cells and muscle fibers, in a model of MTM. They also found that treatment targeted to the NMJ greatly improved function in the model.
Now, using a research mouse model of MTM, the study team will examine the relationship between the NMJ and the disease. In addition, the investigators will test a commonly used NMJ-modifying drug in the model to determine whether it affects disease progression.
Findings from this work are expected to significantly advance understanding of the MTM disease process, and may lead directly to treatment for people with the disease.
"MDA has been and continues to be a critical funding source for my research," Dowling said. "My work in this area of study started with support from the MDA in the form of an MDA development grant. Using the funding from that grant, I was able to make major inroads in terms of our understanding of MTM. This work also provided the relevant data from which the hypothesis for our new study evolved.
"We believe the results of this study, funded by the MDA, will lead to the first therapy for MTM."
Funding for this MDA grant began February 1, 2011.
Grantee: MTM - James Dowling, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MMD - Mani Mahadevan, M.D.

MDA has awarded a research grant totaling $435,000 over three years to Mani Mahadevan, professor in the department of pathology, medical director of the molecular diagnostics lab and associate director of the cytogenics lab at the University of Virginia in Charlottesville. The new funds will help support Mahadevan’s study of the underlying molecular mechanisms in type 1 myotonic muscular dystrophy (MMD1, or DM1).
MMD1 is caused by an expanded section of DNA in the DMPK gene that results in the accumulation of mutated DMPK RNA (the chemical step between DNA instructions and protein production) in the cell’s nucleus. These "RNA foci" are thought to lead to RNA toxicity by affecting the function of RNA binding proteins that interact with the toxic RNA, disrupting normal RNA processing.
Using muscle cells taken from research mouse models and MMD1-affected individuals, Mahadevan and colleagues plan to examine the interactions in MMD1 between a number of particular proteins and DMPK RNA.
Mahadevan’s work potentially may lead to a greater understanding of the molecular mechanisms that drive MMD1, which will further inform researchers’ efforts to design therapeutics to combat the disease.
"MDA has funded research in my lab since 1998, and often has been the first agency to fund our research at its earliest stages," Mahadevan said. "I am sincerely grateful for the trust and confidence the Association has placed in us and our research."
Funding for this MDA grant began February 1, 2011.
Grantee: MMD - Mani Mahadevan, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MMD - John Lueck, Ph.D.

MDA has awarded a research development grant totaling $180,000 over three years to John Lueck, a postdoctoral fellow at the University of Iowa Carver College of Medicine in Iowa City. The new funds will help support Lueck’s research into the mechanisms responsible for muscle weakness and degeneration in type 1 myotonic muscular dystrophy (MMD1, or DM1).
Although a number of bodily systems are affected in MMD1, likely due to flaws in a number of molecular pathways, and while some progress has been made in understanding certain of these areas, the particular mechanism that underlies muscle weakness and degeneration in the disease has yet to be elucidated.
Lueck and colleagues plan to use a multifaceted approach, using research mouse models of MMD1 and tissue taken from individuals with MMD1 to zero in on the specific molecular flaws responsible for muscle weakness and degeneration, as well as the mechanisms through which those flaws work. Such understanding is crucial for both understanding the MMD1 disease process and for pinpointing targets at which potential therapeutics may be aimed.
"As I am in the early career stages of becoming an independent investigator, the process of obtaining MDA funding has been crucial for gaining experience in grantsmanship, and securing funding for my work," Lueck said. "Importantly, MDA is promoting basic and applied research to not only better understand normal function but also mechanisms of muscle disease and therapy development."
Funding for this MDA grant began February 1, 2011.
Grantee: MMD - John Lueck, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MMD - Araya Puwanant, M.D.

MDA has awarded a clinical research training grant totaling $173,400 to Araya Puwanant at the University of Rochester (New York) Medical Center. The new funds will support completion of a two-year fellowship during which Puwanant will study the disease process in myotonic muscular dystrophy (MMD, or DM).
"In recent years there has been progress in understanding why people with MMD develop muscle weakness and stiffness," Puwanant said. "As more is being learned about the disease, scientists are making progress in developing experimental treatments."
Some therapies currently under development likely will move from laboratory testing to testing in human clinical trials.
"In preparation for clinical trials, we need to have detailed information about how the condition changes over time," said Puwanant. "We also need to find good ways to measure whether new treatments are having a beneficial effect."
Puwanant will be taking part in research studies to carefully chart the progression of MMD over time in a large group of individuals with the disease. Analysis of these observations is expected to help to determine the best way to measure the severity of MMD. Additionally, information gained from Puwanant’s study may help determine the effectiveness of future experimental treatments for the disease.
Funding for this MDA grant began February 1, 2011.
Grantee: MMD - Araya Puwanant, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MG - Pragna Patel

MDA has awarded a research grant totaling $121,139 to Pragna Patel, professor at the Institute for Genetic Medicine, Keck School of Medicine, at the University of Southern California in Los Angeles. The new funds will help support Patel’s research aimed at determining the faulty gene responsible for myasthenia gravis (MG) in a particular family with the disease.
Although MG tends to be sporadic, Patel and colleagues have identified a family in which 10 individuals in two consecutive generations are affected with the disease. The study team has narrowed down the region of DNA in which the gene responsible for these familial, or inherited, cases is likely located; using state-of-the-art technology the team plans to identify not only the gene, but genetic factors that operate in the same cellular pathway or through similar means.
"The typical person with MG has no family history of the disease, thus making it very challenging to identify genes responsible for causing the disease," Patel said. "The knowledge gained from studying the rare family with MG could potentially be used to identify genetic factors that act in the same cellular pathway, or by a similar mechanism, in all or most cases of MG.
Findings from Patel’s study could uncover biological targets at which scientists could aim future therapies.
"MDA funding is vital to our ability to accomplish our goal of finding MG-associated genes," Patel said, noting previous support from the Association has allowed her to conduct a number of studies and add to the collective knowledge pool surrounding MG and also type 1 Charcot-Marie-Tooth disease (CMT1A).
Funding for this MDA grant began February 1, 2011.
Grantee: MG - Pragna Patel
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MG - Jon Lindstrom

MDA has awarded a research grant totaling $450,000 over three years to Jon Lindstrom, professor of neuroscience and pharmacology at the Medical School of the University of Pennsylvania in Philadelphia. The new funds will help support Lindstrom’s continued efforts to develop an immunosuppressive therapy for myasthenia gravis (MG).
MG is caused by an immune system attack, via antibodies, on acetylcholine receptors in muscle. The attack leads to the degradation and loss of the acetylcholine receptors, disrupting communication between nerves and muscles. The cause of the disease is unknown.
Lindstrom and colleagues are working on the continued development of a specific immunosuppressive therapy for MG, which they developed using a rat research model of the disease. The team will continue testing the therapy while working in parallel to determine the mechanisms by which it works.
Current therapies for MG compensate for the loss of acetylcholine receptors by using inhibitors to block the enzyme that destroys acetylcholine, along with drugs that cause massive suppression of all, or nearly all, immune responses.
In contrast, the therapy under development by Lindstrom's team is designed to target only the immune system attack on acetylcholine receptors. Such a therapy is expected to be more effective than those in use now and likely would preclude some of their known side effects.
Results of Lindstrom’s work may lead to further insight into the MG disease process and inform researchers’ efforts at therapy development.
"Support from the MDA has been important to my career from its very beginning when we first discovered EAMG and made progress in understanding its nature," Lindstrom said. "Hopefully, this new support from the MDA will help us go full circle, from helping to discover the autoimmune nature of the disease to helping to discover how to specifically suppress the autoimmune response in MG."
Funding for this MDA grant began February 1, 2011.
Grantee: MG - Jon Lindstrom
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MG - Chi Wai Lee

MDA has awarded a research development grant totaling $180,000 over three years to Chi Wai Lee, postdoctoral fellow in the department of cell biology at Emory University School of Medicine in Atlanta. The grant will help support Lee’s research into therapeutic strategies to ameliorate the symptoms in myasthenia gravis (MG).
In MG, the immune system attacks the acetylcholine receptors responsible for nerve-to-muscle communication, leading to muscle weakness due to the loss of nerve signals. Lee’s team plans to uncover the mechanisms underlying the attack and subsequent loss of acetylcholine receptors.
Using frog and mouse MG research models, Lee and colleagues plan to examine the molecular and cellular mechanisms underlying proper functioning of the neuromuscular junction (NMJ), the place where nerve meets muscle and where acetylcholine receptors do their job.
Results from Lee's work are expected to provide increased understanding of the molecular and cellular mechanisms underlying degradation and loss of acetylcholine receptors in the NMJ. In addition, data analysis may point the way to new therapies designed to ameliorate MG symptoms by targeting the mechanisms underlying acetylcholine receptor loss.
"I am very grateful to receive this MDA development grant at the early stage of my scientific career," Lee said. "The support from MDA will allow me to extend my current research and training to become an independent researcher focusing on the study of neuromuscular diseases."
Funding for this MDA grant began February 1, 2011.
Grantee: MG - Chi Wai Lee
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MD - Xiaohua Wu, M.D., Ph.D.
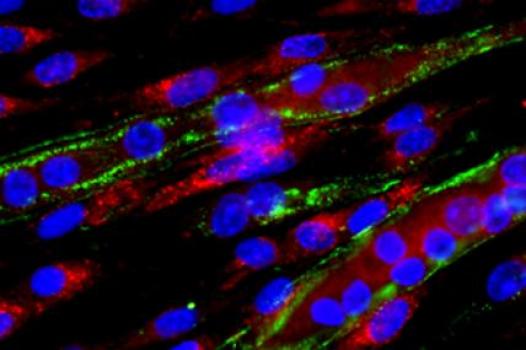
MDA has awarded a research grant totaling $224,863 over three years to Xiaohua Wu, research faculty for the drug discovery group at the McColl-Lockwood Laboratory for Muscular Dystrophy Research at Carolinas Medical Center in Charlotte, N.C. The new funds will help support Wu’s research into effective therapies for various types of muscular dystrophy (MD).
The majority of muscular dystrophies are associated with compromised linkages between key structures due to various genetic defects, Wu said. The linkages play crucial roles in muscle function, and strengthening them by increasing the activity of certain genes has proven useful in MD mouse models.
In his new work, Wu and colleagues plan to screen more than 100,000 small compounds and identify those that might help enhance muscle function in various MDs.
The team will then test the therapeutic potential of the identified compounds in cell culture and MD mouse models.
Findings from Wu's work are expected to speed development of drugs for treatment of a number of muscular dystrophies.
"The new MDA grant is essential for our project," Wu said, "allowing us to gather enough resources to conduct the research necessary for developing drugs for MDs."
Funding for this MDA grant began February 1, 2011.
Grantee: MD - Xiaohua Wu, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – MC - Mark Rich, M.D., Ph.D.

MDA has awarded a research grant totaling $53,358 to Mark Rich, an associate professor at Wright State University in Dayton, Ohio. The new funds will help support Rich’s study of the disease process in myotonia congenita (MC).
MC is an inherited muscle disease in which muscle is stiff because it contracts too much. The cause of stiffness is a mutation in a protein that is involved in electrical signaling in muscle.
Rich and colleagues plan to study a particular clinical feature of MC: While patients exercise, their muscle stiffness lessens. The effect is temporary and reverses after exertion. The team will work with a mouse model of MC that, like humans with the disease, experiences muscle stiffness that decreases with exercise.
Rich’s goal is to determine the molecular mechanisms underlying the exercise effect in MC. His team hypothesizes that a single protein may be responsible for "turning on" during exercise and causing the reduction in stiffness. If findings from the work identify such a protein, then it potentially could be targeted and manipulated into turning on all the time, providing symptomatic relief to individuals with the disease.
The investigators will test the drug in the mouse and observe whether it reduces their stiffness.
"MDA funding is critical to the success of this project," Rich said.
Funding for this MDA grant began February 1, 2011.
Grantee: MC - Mark Rich, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – LGMD - Peter Kang, M.D.

MDA has awarded a research grant totaling $299,722 over three years to Peter Kang, assistant professor of neurology at Harvard Medical School and director of the electromyography laboratory at Children’s Hospital Boston. The funds will help support Kang’s research into identification of gene mutations that can cause limb-girdle muscular dystrophy (LGMD).
"More than 18 different genes have been linked to various forms of LGMD," Kang said. "Each subtype is rare, but in combination the LGMDs comprise a major class of inherited muscle diseases."
Kang and colleagues have gathered and analyzed DNA samples from a number of families affected by LGMD. The investigators will use both the traditional technique called "linkage analysis," and the newer "whole-genome sequencing" to find the new genes, after which they plan to determine which of those identified are likely to be causative mutations.
Whole-genome sequencing is expected to become widely available, probably on a clinical or even direct-to-consumer basis, within a few years, Kang noted. The major question that will surround the enormous volume of data will be clinical interpretation. This project will illustrate how whole-genome sequencing results can be meaningful for individual patients, and may serve as an example of how they can be applied in non-research settings.
The identification of additional LGMD-related genes is expected to expand scientists’ knowledge of muscle disease processes, and potentially lead to new therapeutic strategies for a number of LGMDs and other muscular dystrophies.
"The importance of MDA funding for a project such as this is enormous," Kang said. "MDA is one of the most significant funding agencies for research in this field, and has had a major impact on advancing our knowledge of neuromuscular diseases."
Funding for this MDA grant began February 1, 2011.
Grantee: LGMD - Peter Kang, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – LGMD - Michael Hauser, Ph.D.
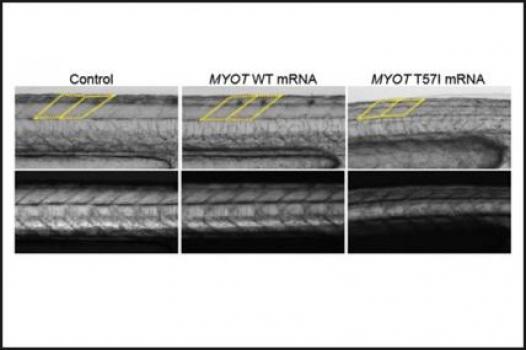
Michael Hauser, an associate professor in the Section of Medical Genetics, Department of Medicine at Duke University Medical Center in Durham, N.C., has been awarded an MDA grant totaling $383,856 over a period of three years. The funds will help support Hauser's efforts to identify mutations responsible for dominantly inherited (type 1) limb-girdle muscular dystrophy (LGMD). (Dominant inheritance means only one gene mutation, inherited from one parent, is sufficient to cause the disease. As a result, offspring of affected individuals have a 50 percent probability of inheriting the condition from their parents.)
Although a number of genes have been associated with various types of LGMD, others remain as yet unidentified. Hauser and colleagues plan to uncover additional mutations responsible for type 1 LGMD.
To do this, the investigators will examine DNA samples collected from 18 families affected by dominantly inherited LGMD. They plan to use a sophisticated technique called whole exome sequencing to probe the genetic blueprints of the affected individuals and compile a list of possible LGMD-causing mutations. Finally, the team will test the mutations in a zebra fish research model of LGMD in order to determine which of them interfere with muscle function.
"Zebra fish are perfect for this approach, because they incorporate the mutated proteins into their own muscles and can be analyzed in just a few days," Hauser said. "The combination of whole exome sequencing and functional testing in the zebra fish is extremely powerful, and we hope to identify several new causes of muscular dystrophy in this way."
MDA funding, Hauser said, has played an "extremely important" role in his career.
Funding for this MDA grant began February 1, 2011.
Grantee: LGMD - Michael Hauser, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – LEMS - William Atchison, Ph.D.

MDA awarded a research grant totaling $336,503 to William Atchison, professor of pharmacology/toxicology and acting dean for research at Michigan State University College of Veterinary Medicine in East Lansing. The funds will support Atchison's research into the biological mechanisms underlying Lambert-Eaton myasthenic syndrome (LEMS).
In LEMS, the immune system mistakenly mounts an attack, targeting proteins called "types P and Q calcium channels" located at the neuromuscular junction, the place where motor neurons (nerve cells) and muscles meet. These proteins regulate the release of acetylcholine, the chemical messenger that carries signals between the motor neuron and the muscle.
In previous MDA-supported work, Atchison found that mice treated with plasma taken from people with LEMS reacted to an attack on the P/Q calcium channels by recruiting members of other classes of calcium channels (types L and R) in an effort to compensate for loss of the P/Q types.
In his new work, Atchison plans to study the compensation strategy and determine how muscle-controlling nerve cells try to maintain their function via recruitment of the L and R types of calcium channels.
Atchison's work could pave the way to a gene therapy strategy based on increasing the availability of compensating proteins as a means of preventing disruption of the chemical messenger.
"MDA funding has been crucial in that it has allowed us to study a relatively rare neuromuscular disease, but one that has very severe consequences, and that affects the quality of life and its length in patients with LEMS," Atchison said. "It has also allowed us to focus on aspects of this disease that might help increase general understanding of how the neuromuscular system tries to compensate for damage in other forms of autoimmune disease."
Funding for this MDA grant began February 1, 2011.
Grantee: LEMS - William Atchison, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – IBM - Steven Greenberg, M.D.
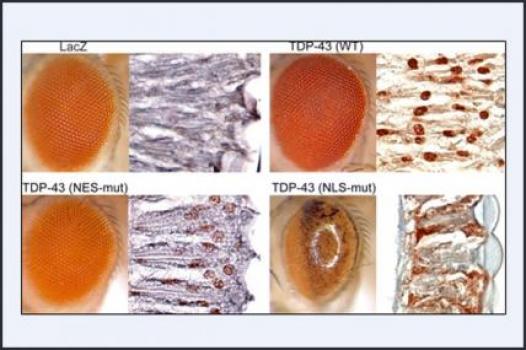
MDA has awarded a grant totaling $444,314 over a period of three years to Steven Greenberg at Brigham and Women's Hospital and Harvard Medical School in Boston. The funds will help support Greenberg's study of the role of a protein called TDP43 in sporadic and hereditary inclusion-body myositis (IBM).
In recent studies conducted on muscle fibers taken from people with IBM, researchers have found that a significant amount of TDP43 protein moves from its normal position inside the cell nucleus to the main part of the muscle fiber called the sarcoplasm. It's also known that TDP43 binds to RNA (the chemical step between DNA and protein synthesis), making it possible that the protein's redistribution in IBM disrupts the function of important RNA molecules in muscle sarcoplasm.
Greenberg and co-principal investigator J. Paul Taylor, M.D., Ph.D., at St. Jude Children's Research Hospital in Memphis, Tenn., plan to examine the effects of TDP43 redistribution to the sarcoplasm, with a particular focus on whether it causes damage to muscle fibers. (Taylor previously has demonstrated that similar mislocation of TDP43 is toxic in the eye of a fruit fly research model.) The researchers also plan to identify the specific RNA molecules affected by the mislocated TDP43.
MDA funding is critical, Greenberg said, because, "The Association funds translational discovery research, placing a priority on relevance to people with disease."
Funding for this MDA grant began February 1, 2011.
Grantee: IBM - Steven Greenberg, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – FA - Joseph Sarsero, Ph.D.

MDA has awarded a research grant totaling $416,250 over three years to Joseph Sarsero, head of the Friedreich Ataxia Laboratory Research Program at the Murdoch Childrens Research Institute in Parkville, Victoria, Australia. The new funds will help support Sarsero’s development of a new mouse model of Friedreich’s ataxia (FA).
Prior to evaluating new therapies in people with FA, it is important that they be tested in appropriate biological models of the disease, Sarsero noted.
"Animal models that are generated by the 'knocking out' of specific genes often manifest the main symptoms of the corresponding human disorder; however, such models rarely recapitulate the precise molecular cause that underlies the human disease."
Accurate "humanized" mouse models of disease are designed to contain an entire human gene of interest and harbor the specific disease-causing mutations found in people with the disease. Such mice should not only manifest the main symptoms of a disorder, but also provide the correct underlying molecular cause of the disease.
Utilizing information and resources generated as part of the Human Genome Project, coupled with their expertise in handling the FA gene and their preliminary mouse models of the disease, Sarsero and colleagues plan to generate an improved humanized FA mouse model that will contain the entire human FA gene.
Funding for this MDA grant began February 1, 2011.
"The mouse model will more accurately reflect the underlying molecular cause of FA and exhibit the key molecular, biochemical, epigenetic and behavioral traits of the disorder," Sarsero said.
This new research tool is expected to enable scientists to better understand the molecular underpinnings of FA as well as provide a better subject in which to test potential FA therapeutics.
"MDA support has been pivotal in allowing us to pursue major projects aimed at identifying new pharmacological therapies for Friedreich's ataxia and the generation of accurate mouse models of the disorder," Sarsero said.
Grantee: FA - Joseph Sarsero, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – FA - Giovanni Coppola, M.D.

MDA has awarded a research grant totaling $398,541 to Giovanni Coppola, assistant professor and co-director at the University of California, Los Angeles, Informatics Center for Neurogenetics and Neurogenomics. The funds will help support Coppola's research into biological indicators, called "biomarkers," in Friedreich's ataxia (FA).
In a recent pilot study, Coppola and colleagues used microarray technology (a technique that allows researchers to check the gene "expression," or activity, level of thousands of genes at the same time) to identify a set of 77 genes that exhibited changes that correlated with disease status in 10 FA-affected individuals, 10 healthy volunteers and 10 FA "carriers," (people who carry only one defective copy of the gene responsible for FA).
In his new work, Coppola plans to validate the 77 previously identified genes by studying a 10-times larger group of subjects over a period of three years.
Coppola hopes to identify a "signature" of disease that can be used in clinical trials to monitor disease progression and the effects of experimental treatments.
"MDA funding will allow me to test an innovative approach to identifying biomarkers in Friedreich's ataxia, which can potentially be applied to a number of other neurogenetic diseases as well," Coppola said.
In parallel with its biomarker studies, Coppola's team plans to collect genetic information, including gene expression data, from study participants. The researchers will store the data in a large Web-based database, providing the scientific community with the first centralized repository of gene expression data in FA.
Funding for this MDA grant began February 1, 2011.
Grantee: FA - Giovanni Coppola, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD/BMD - Masahiko Hoshijima, M.D., Ph.D.
MDA awarded a grant totaling $367,386 over three years to Masahiko Hoshijima, professor of medicine at the University of California, San Diego in La Jolla, Calif. The funds will help support Hoshijima's research into connections between cardiac and respiratory failure in muscle diseases such as Duchenne (DMD), Becker (BMD) and other muscular dystrophies (MDs).
Hoshijima plans to study how heart failure and respiratory failure are interlinked in MD. Weakness of the respiratory muscles, including the diaphragm and intercostal (part of the rib cage) muscles, leads to inadequate breathing called "hypoventilation" in many MDs. In contrast, these diseases affect the heart muscle directly.
Using the BIO14.6 hamster research model, Hoshijima and colleagues will administer gene therapy selective for muscle type at two different time points: early-stage treatment meant to prevent disease onset and progression, and later treatment meant to "rescue" animals after heart and respiratory system damage is well-developed.
One group of animals will receive heart treatment only; a second group will be treated with a therapy that targets skeletal and respiratory muscles and excludes heart treatment. The study team hopes data from these studies can clarify how heart failure and respiratory failure are independently as well as collaboratively contributing to the ultimate disability and death of the animal model of muscular dystrophy.
In further studies, the investigators plan to administer muscle-specific gene therapy to the rodent model in order to determine whether heart treatment can improve respiratory function and whether skeletal muscle-specific gene therapy can benefit systemic circulation.
Findings from Hoshijima's work may provide a better understanding of how better to treat human muscular dystrophies that affect more than one muscle type.
MDA funding, Hoshijima noted, is "vital to select rare-disease patient groups who can find hope in the potential for development of therapies tailored to their diseases."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD/BMD - Masahiko Hoshijima, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD/BMD - Jianming Liu, Ph.D.
MDA has awarded a research development grant totaling $179,912 over three years to Jianming Liu in the department of cell and tissue biology at the University of California, San Francisco. The new funds will help support Liu’s study of the disease process in Duchenne (DMD) and Becker (BMD) muscular dystrophies. Using research mouse models for DMD and BMD, Liu and colleagues plan to investigate a potential stem-cell-based therapy for the two diseases using engineered stem cells designed to help regenerate healthy skeletal muscle tissues.
One of the hallmarks of muscular dystrophy is the rapid depletion of immature muscle cells due to repeated cycles of muscle degeneration and regeneration, Liu noted. Therefore, using normal, healthy stem cells to promote muscle regeneration represents a possible therapeutic approach.
Liu and colleagues will investigate the potential of cell-based therapy for muscular dystrophy using engineered stem cells with modified cell signaling capabilities designed to prompt regeneration of healthy skeletal muscle tissues. Findings from Liu’s work are expected to help increase understanding of the potential for muscle regeneration in the muscular dystrophies as well as provide testing data for potential therapeutics.
"I'm currently a postdoc but am in the process of transitioning into an independent researcher able to lead my own research team and tackle the challenges of finding cures for devastating muscle diseases; for that reason, MDA support at this time is essential and pivotal for my career development and for making fast progress in my research," Liu said. "I am greatly appreciative of the opportunity and the support MDA has provided me.
Funding for this MDA grant began February 1, 2011.
Grantee: DMD/BMD - Jianming Liu, Ph.D.
Grant type: Development Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD/BMD - Harold Bernstein, M.D., Ph.D.
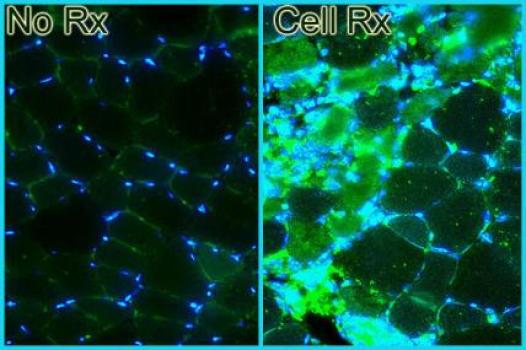
Harold Bernstein, professor of pediatrics at the University of California, San Francisco, has been awarded an MDA research grant totaling $540,000 over three years. The award will help support Bernstein's study of human muscle development and potential cell-based therapies for treatment of degenerative muscle diseases such as Duchenne muscular dystrophy (DMD).
In his new work, Bernstein and colleagues will study the pathways that different types of muscle stem cells follow as they form new muscle, and identify the particular muscle stem cell types that appear most suited for therapeutic development.
The team will first observe the maturation of human stem cells into muscle cells in culture (in the laboratory), as a means of identifying the stages of normal muscle development. The investigators will then transplant the cells at various stages of development into the leg muscles of mice with a disease resembling DMD and study the process by which these cells become new muscle tissue, how this affects the animals' ability to exercise, and the strength of the treated muscles.
Bernstein's team hopes to fully elucidate the process of normal human muscle stem cell development and, in addition, identify specific stem cell types that may provide therapeutic benefit when transplanted into DMD-affected muscle.
"Funding from the Muscular Dystrophy Association will allow us to extend our research on muscle development and stem cell biology into studies specifically focused on therapy," Bernstein said. "This funding provides important support for research that will directly translate into improved treatment for patients with muscular dystrophy."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD/BMD - Harold Bernstein, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD/BMD - David Kass, M.D.

MDA has awarded a research grant totaling $210,618 over two years to David Kass, professor of cardiology, medicine and biomedical engineering at Johns Hopkins University in Baltimore. The new funds will help support Kass’ study of the disease process and potential therapeutic strategies in Duchenne (DMD), Becker (BMD) and other muscular dystrophies.
One particular problem in DMD is hyperactive "TRPC" channels in the muscle cell membrane that leak calcium and sodium. These channels can increase calcium levels inside the cell, stimulating oxidative stress, which can lead to cell damage and/or death.
Kass and colleagues recently discovered that a protein called PKG (for protein kinase G) can directly suppress TRPC channels.
Kass plans to test the importance of PKG activation to block stimulated TRPC channels in both heart muscle and skeletal muscle cells in research models of muscular dystrophy, and determine if such modification can inhibit muscle damage associated with excess calcium and oxidative stress.
Findings from Kass’ work may inform development of a treatment based on the pathways relevant to TRPC channel regulation. A number of drugs already approved for treatment of other conditions by the U.S. Food and Drug Administration (including sildenafil, or Viagra) hold potential for this type of treatment.
"It's great to become part of the MDA family, and I am very excited about pursuing the work we are undertaking," Kass said.
Funding for this MDA grant began February 1, 2011.
Grantee: DMD/BMD - David Kass, M.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD/BMD - Andrew Brack, Ph.D.

Andrew Brack, an assistant professor at the Center for Regenerative Medicine at Massachusetts General Hospital in Boston, was awarded an MDA research grant totaling $353,259 over three years. The funds will help support Brack's research into the function of adult muscle stem cells called "satellite cells" in Duchenne muscular dystrophy (DMD) and possibly in other muscular dystrophies as well.
Satellite cells are responsible for tissue maintenance and repair over a lifetime. It's widely accepted among scientists that these cells are sufficient to promote muscle repair; however, it is unknown what is required of them for regeneration of diseased skeletal muscle and whether the number and function of cells in the satellite cell pool can be manipulated as a means of slowing or ameliorating disease progression.
Brack and colleagues plan to use targeted genetic approaches to manipulate the number and function of adult satellite cells in a research mouse model of DMD. From this they hope to gain an understanding of the relative importance of both number and functionality of satellite cells needed for effective muscle repair.
Results from Brack's work may shed light on new therapeutic strategies for slowing disease progression that are based on retaining a functional pool of satellite cells in people with DMD and other muscular dystrophies.
"Without funding through MDA," Brack said, "this project would not have been realized."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD/BMD - Andrew Brack, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD - Young-Jin Son, Ph.D.
MDA has awarded a research grant totaling $375,000 over three years to Young-Jin Son, associate professor of developmental neurobiology at Temple University in Philadelphia. The new funds will help support Son’s study of nerve and muscle interaction in muscle diseases such as Duchenne muscular dystrophy (DMD).
Stable maintenance of muscle size and also the connections between nerves and muscles are critical for normal neuromuscular function in adulthood, Son said, noting disruption of the balance plays a central role in the initiation and progression of numerous neuromuscular disorders.
Son and colleagues plan to study whether cellular structures called "muscarinic acetylcholine receptors" (mAChRs) comprise a novel receptor system that is used by nerve cells and muscles to monitor nervous system activity, and to maintain stable nerve-muscle connections and solid muscle mass.
The investigators also will explore the possibility that muscle atrophy can be prevented via treatments that directly target muscarinic receptors. Our studies will provide insights that may prevent synaptic loss and muscle atrophy, and has the potential to develop new strategies for treating a broad range of neuromuscular disorders.
Results from Son’s work may provide leads on the development of new strategies for treating a broad range of neuromuscular disorders.
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Young-Jin Son, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
MDA Resource Center: We’re Here For You
Our trained specialists are here to provide one-on-one support for every part of your journey. Send a message below or call us at 1-833-ASK-MDA1 (1-833-275-6321). If you live outside the U.S., we may be able to connect you to muscular dystrophy groups in your area, but MDA programs are only available in the U.S.