Search our Grants
MDA’s research program awards grants to the world’s best scientists investigating promising theories and therapies that may accelerate treatments and cures for families living with muscular dystrophy, ALS and related neuromuscular diseases.
Grant – Winter 2011 – DMD - Pier Lorenzo Puri, M.D., Ph.D.
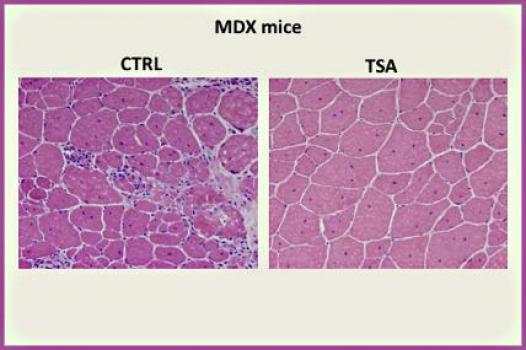
MDA has awarded a research grant totaling $309,336 over three years to Pier Lorenzo Puri, professor of pediatrics at the University of California, San Diego, and associate professor of muscle development and regeneration at the Sanford-Burnham Medical Research Institute, both in La Jolla, Calif. The funds will help support Puri's study of the molecular underpinnings of, and the identification of treatments for, Duchenne muscular dystrophy (DMD).
Puri and colleagues plan to examine the regulation of skeletal muscle regeneration at the molecular level, with a particular focus on various regeneration signals and the ways in which different cellular populations in the regenerative environment activate distinct programs of gene expression (activity).
This knowledge is instrumental, Puri said, to gaining an understanding of the molecular and functional interactions between the cellular components of the muscle stem cell population (satellite cells), and identification of targets at which to aim selective interventions that will promote muscle regeneration and stave off the invasion of fibrosis (scarring) and fatty tissue.
To do this, the investigators first will isolate and manipulate muscle-derived cell populations from mouse models of muscular dystrophies; then they'll determine those cells' molecular, epigenetic (gene regulation) and functional characteristics.
Data obtained from Puri's studies is expected to inspire and drive new pharmacological strategies suitable for speedy translation into treatments for muscular dystrophies and other neuromuscular diseases. The study team will prioritize potential interventions based on strategies or compounds that can be translated most quickly into clinical trial testing.
"I have been supported by MDA since my early career stages with a development grant, through my current position as an established investigator with a primary research grant," Puri said. "This support helped me maintain the focus of my interest and efforts on the disease process and treatment of muscular dystrophies."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Pier Lorenzo Puri, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD - Jen-Chywan Wang, Ph.D.

MDA has awarded a research grant totaling $220,000 over two years to Jen-Chywan Wang, assistant professor at the University of California, Berkeley. The new funds will help support Wang’s study of the effects of chronic glucocorticoid (steroid) treatment in Duchenne muscular dystrophy (DMD).
Glucocorticoids are potent anti-inflammatory agents that frequently are used to delay and relieve DMD symptoms, including rapid progression of muscle degeneration, loss of ambulation, paralysis and shortened lifespan. Although the drugs are beneficial, they cause unwanted side effects, one of which is loss of muscle mass.
In their new work, Wang and colleagues plan to first investigate the means by which glucocorticoids cause muscles to degenerate. The investigators previously identified two genes that have been linked to reduced muscle mass; now they plan to examine how glucocorticoids increase those genes' activity ("expression").
The team also plans to identify chemical compounds that specifically suppress the ability of glucocorticoids to activate the two suspect genes without affecting the drugs' anti-inflammatory activity. In research models of the disease, Wang's team will test whether such chemical compounds can reduce inflammation without causing muscle loss.
Additionally, the investigators have identified another eight genes that exert control over the total amount of protein in cells. The group will test whether increasing the expression of these genes leads to muscle loss, and whether the genes are responsible for glucocorticoid-induced muscle loss. Such genes could potentially be targeted in an effort to improve the effectiveness of glucocorticoid treatment in DMD.Wang’s work could lead to improved glucocorticoid-based therapies for individuals with DMD.
"Private agencies such as MDA play a vital role in sustaining specific interest in particular diseases," Wang said. "This support from MDA will provide a tremendous boost to our research in the understanding of how steroid hormones affect skeletal muscle biology."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Jen-Chywan Wang, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD - Hao Shi, Ph.D.

MDA has awarded a research development grant totaling $180,000 over three years to Hao Shi, associate research scientist at Yale University in New Haven, Conn. The new funds will help support Shi’s study of muscle repair and regeneration in Duchenne muscular dystrophy (DMD).
Shi plans to study the functional role of a protein called mitogen-activated protein kinase (MAPK) phosphatase-5, or MKP-5, in DMD. It is known that muscle repair and regeneration are dependent on the MAPKs. However, little is known about how the regulatory pathways that inactivate the MAPKs control muscle repair or whether they are involved in the disease process in skeletal muscle disorders.
Using a mouse model of DMD, Shi and colleagues plan to elucidate the role of MPK-5 in skeletal muscle. They will study the protein's role in muscular dystrophy; define its role in skeletal muscle regeneration; and identify the molecular mechanisms of MKP-5 in muscle stem cell function.
Additionally, the team will use a mouse model engineered to be deficient in MKP-5 to study the process of post-development muscle regeneration.Results from Shi’s studies potentially may reveal one or more biological pathways involved in muscle regeneration at which scientists might target new therapeutics in the disease.
"MDA funding not only provides an alternative avenue of financial support to basic science in an exciting and promising field, but it also facilitates the translation of academic research into potentially meaningful clinical outcomes," Shi said. "We believe that these studies will set the foundation from which validation of MKP-5 as a new therapeutic target for the treatment of muscular dystrophy can be established."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Hao Shi, Ph.D.
Grant type: Development Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD - Grace Pavlath, Ph.D.
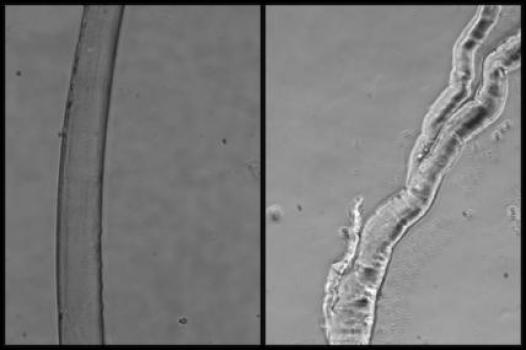
MDA has awarded a research grant totaling $295,269 over three years to Grace Pavlath, professor in the department of pharmacology at Emory University in Atlanta. The new funds will help support Pavlath’s study of abnormal muscle regeneration in the muscular dystrophies, particularly Duchenne muscular dystrophy (DMD).
Pavlath intends to study the abnormal "branched" muscle fibers that sometimes appear as part of the muscle regeneration process after illness or injury. Such fibers have shown characteristic functional abnormalities; they also are weaker and more prone to injury than normal, long cylindrical cells.
Although the exact contribution of branched myofibers to DMD muscle is unclear, muscles containing high levels of branched myofibers are unlikely to function normally. Thus, decreasing the number of branched myofibers likely will be beneficial for improving both muscle physiology, and the efficiency of cell and gene therapy approaches for muscular dystrophy.
Recent work out of Pavlath's lab analyzing the role of MOR23 in muscle regeneration revealed that it's a necessary factor in the proper skeletal muscle regeneration in mice; loss of MOR23 leads to increased myofiber branching.
Using both a healthy mouse and a research mouse model with a DMD-like disease, Pavlath’s team will manipulate two molecules, MOR23 and kirrel12, and examine their roles in regulating myofiber branching in muscle regeneration.
Findings from Pavlath’s work could lead to a clearer understanding of the underlying mechanisms that cause abnormal muscle-fiber branching in regeneration and, in addition, provide biological targets at which to aim future therapies.
"MDA funding has been extremely critical for this and past projects," Pavlath said. "We would not be able to study the mechanisms of myofiber branching without it."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Grace Pavlath, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant – Winter 2011 – DMD - Dongsheng Duan, Ph.D.

Dongsheng Duan, professor in the department of molecular microbiology & immunology at the University of Missouri in Columbia, has received an MDA grant totaling $527,670 over three years. The funds will help support Duan's continued research into gene therapy in Duchenne muscular dystrophy DMD).
DMD is caused by a mutation in the dystrophin gene, which leads to the absence, or near absence, of dystrophin protein.
Duan has worked extensively in the past with adeno-associated virus (AAV) "vectors," the emptied shells of viruses used to encapsulate healthy genes (or modified — in this case, shortened "mini-" or "mirco-dystrophin" genes), which are then injected locally or systemically into research model test subjects undergoing gene therapy treatment.
In previous studies, AAV-mediated micro-dystrophin gene therapy has shown great promise in ameliorating the symptoms of DMD, and recent developments in systemic AAV delivery suggest the potential for whole-body correction of the flawed dystrophin gene responsible for the disease.
In his latest work, Duan and colleagues plan to test both a new AAV delivery vehicle and a new micro-dystrophin gene, first in a research mouse model of DMD and later in another animal research model of the disease. The investigators will evaluate whether the novel combination ameliorates DMD symptoms.
Findings from this study potentially could accelerate DMD gene therapy research and help advance the strategy to human clinical testing.
"We can now cure a mouse with a DMD-like disease; however, this accomplishment has not been reproduced in people with the disease," Duan said. "The support we receive from MDA is essential for us to move DMD gene therapy forward."
Funding for this MDA grant began February 1, 2011.
Grantee: DMD - Dongsheng Duan, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - SMA — John Manfredi

MDA awarded a grant totaling $79,277 to John Manfredi, chief scientific officer at Sfida BioLogic Inc., in Salt Lake City, Utah, for continued research into new drug compounds that promote the growth and function of motor neurons (nerve cells), and that may have potential as therapeutics for treatment of spinal muscular atrophy (SMA).
Manfredi's research team aims to determine the therapeutic potential of their compounds using a zebra fish research model that has been genetically engineered to simulate aspects of SMA. Results will determine whether the drugs merit future testing in more sophisticated and expensive models of the disease.
The compounds identified by Manfredi and colleagues "exhibit attractive pharmaceutical properties," Manfredi said. "They are not toxic; they penetrate the central nervous system; they exhibit appropriate half-lives in tissues; they can be administered orally; their permeability and solubility characteristics are drug-like; and they can be economically synthesized."
The group also will test a sample of their compounds alongside a collection of biochemically similar, commercially available drugs. If the commercial drugs show effects in the zebra fish model of SMA, the results will support the hypothesis that their SMA-relevant effects are due to shared biochemical activity with the investigators' novel drugs. Positive results also could lead to development of the commercial compounds as SMA therapeutics.
"The funding provided by MDA is absolutely critical for evaluating the potential of our company’s proprietary compounds to treat spinal muscular atrophy," Manfredi said. "Indeed, if these or derivative compounds prove to be efficacious for the treatment of SMA, MDA can legitimately claim responsibility for their success."
Funding for this MDA grant began August 1, 2010.
Grantee: SMA — John Manfredi
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - SBMA — Albert La Spada
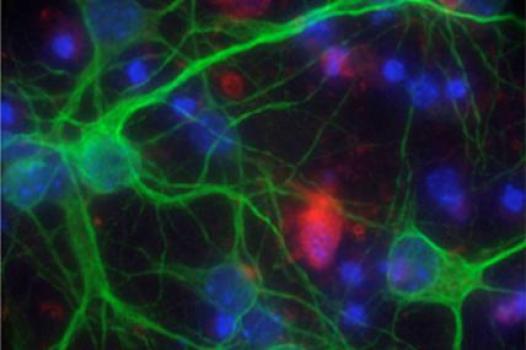
MDA awarded Albert La Spada, chief of the division of genetics in the department of pediatrics at the University of California, San Diego, $330,000 to study what causes nerve cells called motor neurons to die in spinal-bulbar muscular atrophy (SBMA) and other neurodegenerative diseases such as ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease) and spinal muscular atrophy (SMA).
La Spada and colleagues have created mouse and motor neuron cell-culture models of SBMA, which results from a genetic flaw in the androgen receptor (AR) gene. Completed studies have led to the identification of candidate pathways that may spur motor neuron degeneration.
Now, La Spada's team will focus on the androgen receptor (AR) protein, which is biochemically modified and made toxic in SBMA. An understanding of the particular proteins, or "enzymes," that catalyze such chemical modifications will set the stage for identification of drug compounds designed to inhibit them.
The team also will work to identify other cell types involved in driving the SBMA disease process. Using a specialized mouse model, the investigators will "turn off" activity of the mutant, or flawed, AR protein in different cell types, starting with motor neurons and muscle cells, in order to determine whether those cells contribute to making motor neurons sick.
"MDA funding has been critical in allowing us to aggressively pursue cutting-edge research approaches in my lab," La Spada said. "Without MDA, there is no way that we could have made all the progress that we did over the last decade."
Findings from the new work may identify targets for the development of therapies to treat SBMA, ALS and SMA.
Funding for this MDA grant began August 1, 2010.
Grantee: SBMA — Albert La Spada
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - PP — Kurt Beam

MDA awarded a grant totaling $303,438 to Kurt Beam, professor in the department of physiology and biophysics at the University of Colorado School of Medicine in Denver, for research into a process called excitation-contraction coupling responsible for the contraction of muscle cells necessary for voluntary movement and breathing.
"Excitation-contraction coupling is a fundamental process in skeletal muscle that is not yet well-understood," Beam said. "Additionally, mutations of the key proteins involved in excitation-contraction coupling cause serious human muscle diseases, including periodic paralyses, malignant hyperthermia and central core disease."
The process is initiated by electrical impulses which move along the membrane that separates the inside and outside of each muscle cell, and depends, at least in part, on two proteins called DHPR and RyR1.
In completed studies, Beam and his group have shown that mutations of either DHPR or RyR1 affect the behavior of both proteins. In their new work, the investigators will study cultured mouse muscle cells as they attempt to uncover the molecular mechanism for communication between DHPR and RYR1.
"The importance of MDA to neuromuscular research cannot be measured in dollars alone. In particular, MDA has been at the forefront in promoting basic and applied research directed toward understanding normal muscle function, mechanisms of muscle disease and development of therapies for these diseases," Beam said. "An especially important role that MDA plays is to provide funding during early career stages and at times when funding from other sources becomes scarce. I do not believe that I would have been able to establish and maintain my neuromuscular research had it not been for funding from MDA at crucial points in my career."
Funding for this MDA grant began August 1, 2010.
Grantee: PP — Kurt Beam
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - MM - Mito. Myopathy — Leo Pallanck

MDA awarded $312,699 to Leo Pallanck, associate professor of genome sciences at the University of Washington, Seattle, for research into elimination of flawed cell machinery that is the underlying cause of mitochondrial myopathy.
Mitochondria, the tiny "power plants" responsible for producing nearly all of the energy needed for a human cell to function properly, have their own genes — stretches of DNA that carry the genetic instructions used in protein production. There can be hundreds of mitochondria in a single cell, and large cells with particularly high energy demands, such as skeletal and cardiac (heart) muscle cells, may harbor more than 10,000.
Mutated, or flawed, mitochondrial genes lead to flawed mitochondrial proteins that impair the ability of the mitochondria to produce energy and, by extension, the ability of the cell to function.
Recent studies have shown that have a pair of proteins known as PINK1 and Parkin can recognize damaged mitochondria and promote their destruction.
Pallanck and his research team aim to test the hypothesis that PINK1 and Parkin, in conjunction with other cellular factors, can detect and selectively destroy flawed mitochondria, thus alleviating the symptoms associated with mitochondrial mutations by keeping numbers of the defective mitochondria low.
In experiments on a fruit fly model, the investigators will vary the amounts of PINK1 and Parkin, and study the effects on the frequency of mitochondrial mutations and their associated symptoms, including degeneration of muscles and nerves. The work could lead to the development of strategies that can be used to reduce the overall amount of mutation-bearing mitochondria in humans.
"This is my first grant from the Muscular Dystrophy Association, and I cannot overstate the importance of this funding to our work," Pallanck said. "We couldn't perform these studies without support from the MDA."
Funding for this MDA grant began August 1, 2010.
Grantee: MM - Mito. Myopathy — Leo Pallanck
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - MM - Mito. Myopathy — Lee-Jun Wong
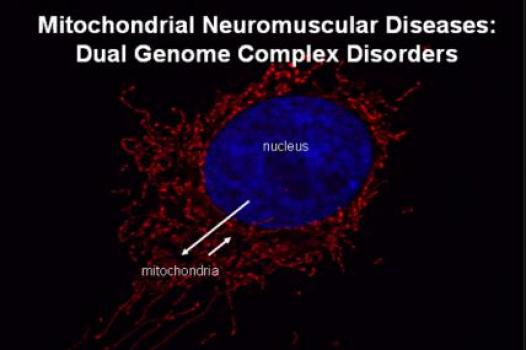
MDA awarded $411,000 to Lee-Jun Wong, professor in the department of molecular and human genetics at Baylor College of Medicine, Houston, for research designed to produce a one-step diagnosis procedure for complex mitochondrial myopathies.
Diagnosis of mitochondrial disorders often is difficult because the disease can present with any of a number of different symptoms including muscle weakness, exercise intolerance, paralysis or weakness of the eye muscles, deafness, seizures, lack of coordination, movement disorder, and strokelike episodes.
Current diagnostic methods are based on the genetic testing of individual genes, one by one. Such a process can be tedious, expensive and time-consuming, and isn't able to uncover all the different types of mutations that may be responsible for mitochondrial disorders.
"The availability of a one-step diagnostic approach is particularly important since mitochondrial myopathy accounts for a large proportion of patients, from children to adults, with muscular dystrophy," Wong said. "Prompt, definitive diagnosis is essential for proper patient management, treatment and genetic counseling."
Wong's group plans to establish a one-step technology that will allow for the detection of all of the various types of mutations that can result in mitochondrial myopathy. The team will validate its technique using archived DNA samples from people with mitochondrial neuromuscular disorders.
"My first research grant was from MDA, and it helped me develop a comprehensive method to screen mutations in mitochondrial DNA," Wong said. "Now, as an established and experienced principal investigator focusing on the molecular diagnosis of mitochondrial disorders, new MDA funding allows me to bring in an innovative one-step approach to comprehensive diagnosis of the complex dual genome mitochondrial disorders."
Funding for this MDA grant began August 1, 2010.
Grantee: MM - Mito. Myopathy — Lee-Jun Wong
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - MMD — Ju Chen
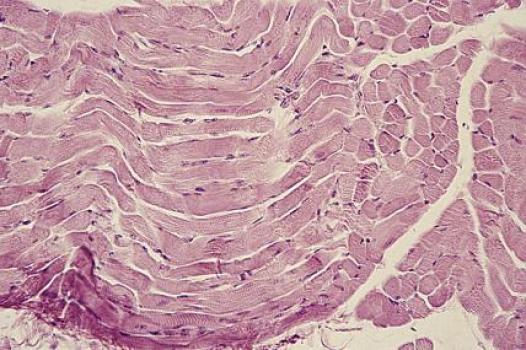
MDA awarded $330,000 to professor of medicine Ju Chen at the University of California, San Diego, for research into the role of a protein called Cypher in skeletal muscle function and disease.
Mutations in Cypher result in myofibrillar myopathy (MFM) and late-onset distal myopathy, and results from completed studies in people with myotonic muscular dystrophy (MMD, or DM) indentified flawed Cypher isoforms (different forms of the same protein) in skeletal muscle tissues. Cypher activity also has been shown to be significantly decreased in mice exhibiting skeletal muscle atrophy.
"These observations suggest that Cypher plays essential roles in skeletal muscle function and disease," Chen said. "A better understanding of the function of Cypher in its various forms is key to developing therapies for Cypher-based MFM and potentially other myopathies."
In its work to understand the role of Cypher in skeletal muscle function and gain insight into the mechanisms by which mutations in Cypher cause skeletal muscle myopathy, the Chen research team will conduct experiments using three different genetically manipulated mouse models: an adult mouse lacking Cypher in skeletal muscle; mice lacking a short form of Cypher; and mice lacking a long form of the protein. Analysis will include comprehensive biochemical and functional evaluation, as well as evaluation of the microscopic structure of cells in skeletal mouse muscle tissue.
"Without funding from MDA, it would not be possible to perform these important studies," Chen said.
Funding for this MDA grant began August 1, 2010.
Grantee: MMD — Ju Chen
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - FSHD — Fedik Rahimov, Ph.D.

MDA has awarded a development grant totaling $180,000 over a period of three years to Fedik Rahimov, a postdoctoral research fellow at the program in genomics at Harvard Medical School and Children's Hospital Boston. The funds will help further elucidate the molecular mechanisms underlyingfacioscapulohumeral muscular dystrophy (FSH, or FSHD).
The mutation associated with FSHD is a contracted (deleted) segment of DNA in a region of chromosome 4 called D4Z4. Although it's not yet fully understood how the contraction causes the disease, it appears to be one of two factors necessary to cause abnormal activation of a gene called DUX4.
The protein produced from the DUX4 gene instructions is toxic to muscle cells, and causes muscle degeneration and weakness.
Fedik and colleagues plan to assess DUX4 activity in FSHD-affected muscles in an attempt to uncover the mechanisms underlying FSHD. It's hoped that understanding and defining these mechanisms may lead to the discovery of disease biomarkers.
"Funding from MDA will help us enormously as we continue our work on biomarker discovery using advanced technologies," Rahimov said. "Data generated from this study will be deposited into public repositories, and the discovery nature of our study will allow the generation of new hypotheses to test for new therapeutic targets."
Funding for this MDA grant began August 1, 2011.
Grantee: FSHD — Fedik Rahimov, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2010 - MMD — Fernando Morales

Fernando Morales, head of the genetic section at the Health Research Institute of the University of Costa Rica, has received an MDA grant totaling $366,210 over three years. The funding will help support Morales’ research to define the molecular underpinnings of type 1 myotonic dystrophy (MMD1, or DM1) and factors that modify the course of this highly variable, multisystem disease.
The MMD1-causing mutation, an expansion in the DNA of a gene on chromosome 19, changes over time, usually becoming larger during a person's lifetime and when being passed between generations. In general, the larger the DNA expansion, the more profound the effects of it are likely to be. However, the correlation between disease severity and age of onset and size of the mutation isn't perfect.
In this project, Morales and colleagues aim to analyze how the MMD1 mutation changes over time and how the changes relate to the clinical course of the disease. They also plan to identify genetic and other modifiers of the MMD1 mutation.
"Importantly," said Morales, "these modifiers of the MMD1 mutation could also be modifiers of the disease, and if so, they could be used as therapeutic targets to delay disease onset and progression."
Morales said he hopes the project will generate more reliable genetic data that can provide more accurate information about prognosis to families and improve disease management and quality of life.
"Funding by MDA is of great importance to our project," Morales said. "Thanks to it, we can investigate in more detail several biological and clinical aspects related to MMD1 in which there is still controversy."
Funding for this MDA grant began August 1, 2010.
Grantee: MMD — Fernando Morales
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - FSHD — Eric Wagner, Ph.D.
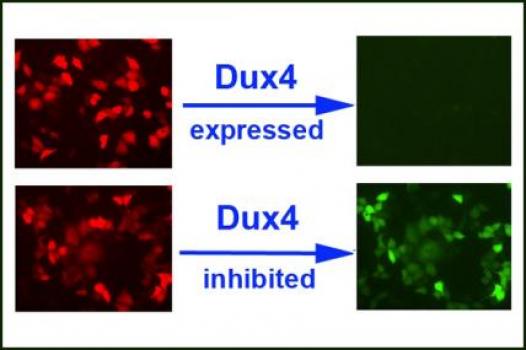
MDA awarded a research grant totaling $284,778 over a period of three years to Eric Wagner, an assistant professor in the department of biochemistry and molecular biology at the University of Texas Health Science Center in Houston. The funds will help support Wagner’s investigations into the role of the DUX4 gene in facioscapulohumeral muscular dystrophy (FSH, or FSHD).
Recent findings implicate the DUX4 gene as a likely causative factor in FSHD and a potential target for molecular therapies. It was found that in people with the disease the normally silent DUX4 gene is inappropriately activated, causing major problems in the muscles.
Wagner and colleagues have developed a reporter system that will monitor their ability to inhibit and study DUX4 expression in normal and FSHD-affected cells.
“The system allows us to design and test inhibitory agents that can antagonize Dux4 expression, which is thought to be the causative agent in FSHD,” Wagner said.
“The value of MDA research funding cannot be underestimated in bringing this research together,” Wagner said. “The recent and critical findings that demonstrated the importance of DUX4’s role in FSHD was funded by MDA, and is what encouraged my lab team to work on this problem.”
Funding for this MDA grant began August 1, 2011.
Grantee: FSHD — Eric Wagner, Ph.D.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2010 - LGMD — Richard Cripps

MDA awarded a grant totaling $339,561 to Richard Cripps, professor and chair of biology at the University of New Mexico in Albuquerque, for research into the role of the Trim32 gene in type 2H limb-girdle muscular dystrophy (LGMD).
The Trim32 gene carries instructions for the important Trim32 protein, required by muscle cells at the right times and in the necessary amounts for muscles to remain healthy and stable.
Cripps and colleagues currently are working to establish a model system in which to study the fruit fly version of Trim32.
"Our research will address fundamental questions about how this gene and its protein specifically behave in both the healthy and muscular dystrophy disease states of muscle," Cripps said. "Finding the answers to these questions will help researchers find critical points of intersection for the future treatment of this form of limb-girdle muscular dystrophy."
In experiments involving the fruit fly model, the team aims to identify the roles and behavior of Trim32 in muscles, including the structures it forms, the role these structures play in tissue stability, and interaction between the Trim32 protein and any other proteins.
"Having been funded by MDA off and on since I was a postdoctoral fellow in 1991-1992, I can deeply appreciate the support that MDA provides to scientists and fellows," Cripps said. "There is a greater willingness in MDA to take a chance on research that is of a higher risk, and the value of this approach has been borne out by the many achievements that MDA funding brings."
Funding for this MDA grant began August 1, 2010.
Grantee: LGMD — Richard Cripps
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - LGMD — Renzhi Han

Renzhi Han, assistant professor of physiology at Loyola University Medical Center in Maywood, Ill., received an MDA grant totaling $405,000 to study mutations in the dysferlin gene that lead to development of several types of muscle diseases known as "dysferlinopathies," including type 2B limb-girdle muscular dystrophy (LGMD).
Previous studies have shown that inflammation, a component of the "innate immune system," is prominent in muscle samples deficient in dysferlin protein. (The innate immune system specializes in providing immediate defense against infection in the body. By contrast, the adaptive immune system specializes in building up protective immunity.)
Han speculates that an attack carried out by the innate immune system might play a role in the onset and progression of muscle diseases that result from dysferlin mutations, and that disabling this immunological response may ameliorate the symptoms of muscular dystrophies caused by dysferlin deficiency.
The investigators will use research mouse models deficient in both dysferlin and the innate immune system to test their hypothesis. The team also will study whether increasing the activity of a biochemical inhibitor of the immune system has any effect on disease progression in dysferlin-deficient mice.
"This research will enable us to accomplish the critical step in our goal to design a therapy for dysferlin-deficient muscular dystrophy," Han said. "This work could not go further without the support of MDA."
Funding for this MDA grant began August 1, 2010.
Grantee: LGMD — Renzhi Han
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - FA — Des Richardson, Ph.D., D.Sc.

MDA has awarded a research grant totaling $625,959 over a period of three years to Des Richardson, professor and senior principal research fellow at the University of Sydney (Australia) School of Medical Sciences. The funds will help support Richardson's continued research into iron metabolism in Friedreich's ataxia (FA).
The aim of Richardson's studies is to examine the role of altered iron metabolism in the cellular energy factories called mitochondria.
Using molecular and cellular models, as well as a research mouse model that closely mimics the neurodegeneration and heart problems observed in people with FA, Richardson and colleagues will test a variety of strategies designed to help elucidate the function of frataxin, the protein that’s deficient in this disease.
The team also will test a new experimental therapy designed to replace or bypass the function of frataxin by getting iron to the mitochondria in a form that can be easily utilized for the processes that are lacking when frataxin levels are inadequate.
"We gratefully acknowledge MDA funding," Richardson said. "The knowledge of these alterations is critical for developing new therapeutics that could replace the function of this molecule in patients."
Funding for this MDA grant began August 1, 2011.
Grantee: FA — Des Richardson, Ph.D., D.Sc.
Grant type:
Award total:
Institution:
Country:
Grant - Summer 2010 - IBM — Virginia Kimonis
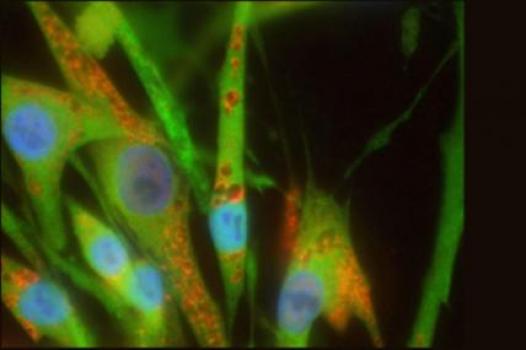
Virginia Kimonis, chief of the division of genetics and metabolism at the University of California, Irvine, received an MDA grant totaling $372,000 to conduct experiments designed to uncover the underlying molecular cause of inclusion-body myopathy associated with Paget's disease of Bone and/or frontotemporal dementia (IBMPFD).
In 2004, Kimonis and colleagues published study results describing the rare disease IBMPFD and identifying the gene responsible for it — valosin-containing protein, or VCP. The group also created a mouse model that carries a flawed human VCP gene and exhibits symptoms similar to those in humans with IBMPFD, including decreased muscle strength and coordination, fatigue, enlarged vacuoles (bubblelike spaces) in the quadriceps muscles, swelling of cellular "energy factories" called mitochondria and abnormal protein clumps.
The Kimonis lab's new project includes the creation of a new mouse model aimed at providing proof of principle that amelioration of IBMPFD symptoms is possible. The team will evaluate functional and biochemical indicators to assess disease progression and effects in these mice. The group also will study whether physical exercise accelerates or decelerates the progression of muscle weakness in the mice, as a means of assessing what effects physical exercise may have on people with IBMPFD.
"Funding from MDA is critical for our laboratory to be able to continue this important work in understanding the key mechanisms related to this progressive and fatal muscle disease," Kimonis said. "Because of its similarities to other muscle disorders such as limb-girdle muscular dystrophy (LGMD), facioscapulohumeral muscular dystrophy (FSHD) and ALS (amyotrophic lateral sclerosis, or Lou Gehrig's disease), it's hoped that this research will advance a deeper understanding of mechanisms and potential treatments across a broad spectrum of neuromuscular diseases."
Funding for this MDA grant began August 1, 2010.
Grantee: IBM — Virginia Kimonis
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - FA — David Lynch, M.D., Ph.D.
MDA has awarded a research grant totaling $202,222 over two years to David Lynch, professor of neurology at Children's Hospital of Philadelphia and University of Pennsylvania School of Medicine. The funds will help support Lynch's study of the relationship between diabetes and Friedreich's ataxia (FA).
Some individuals with FA develop severe cardiomyopathy (heart disease), while others also may develop diabetes and a resistance to the effects of the hormone insulin, Lynch said, noting "This phenomenon may have implications for the overall progression of FA and the mechanisms involved in causing the disease."
Lynch and colleagues plan to examine the relative likelihood of people with FA to develop diabetes. The team will use measurements of blood sugar and blood insulin levels to assess which features of FA make a particular individual more likely to have abnormal insulin resistance.
The team will work to identify specific genes that contribute to abnormal insulin resistance in FA and also determine whether frataxin, the protein that is deficient in FA, contributes to the development of insulin resistance and diabetes.
The MDA grant will “support development of the collaborative group of individuals who will contribute to this project, which leverages previous data also developed with MDA support," Lynch said.
Funding for this MDA grant began August 1, 2011.
Grantee: FA — David Lynch, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - EDMD — Ji-Yeon Shin

MDA awarded $180,000 to Ji-Yeon Shin, a research scientist at Columbia University Medical Center, New York, for continued study of the molecular mechanisms that underlie Emery-Dreifuss muscular dystrophy (EDMD).
"EDMD is an inherited disease of skeletal muscle and heart. Although diagnosis has been improved by the discovery of the most common genetic mutations that cause EDMD, we still have a poor understanding of how these mutations cause muscular dystrophy," Shin said.
Shin's lab previously has discovered that proteins produced from instructions carried by the mutated genes in most cases of EDMD interact with another protein, called LAP1 (lamina-associated polypeptide 1), whose function as yet remains unknown. Shin's team has engineered a new "muscle specific LAP1 knockout" mouse that exhibits symptoms that mimic those in EDMD and has potential therefore to be used as an EDMD research mouse model.
In addition to further characterizing the mouse, Shin's new work will include investigation into how the LAP1 protein affects muscle cell function in mouse and human cultured cells, and in living mice.
The team hopes to generate a better understanding of how specific genetic mutations cause EDMD, which should enable it to identify new processes, such as cell signaling pathways, that could be targets for the development of novel drugs to treat the disease.
"I am very happy to be selected as a Development Grant awardee, and I have great hopes for what I can achieve from the current project funded by MDA," Shin said. "The previous results that have been generated in the last two years built up exciting groundwork. Now the support of MDA will bring this project to the next level and allow us to study cellular mechanisms that underlie the development of Emery-Dreifuss muscular dystrophy."
Funding for this MDA grant began August 1, 2010.
Grantee: EDMD — Ji-Yeon Shin
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - EDMD — Howard Worman

MDA awarded a grant totaling $310,893 to Howard Worman, professor of medicine and pathology, and cell biology at Columbia University Medical Center in New York, for research into treatments that target the underlying cellular disease process responsible for heart damage, or "cardiomyopathy," in people with Emery-Dreifuss muscular dystrophy (EDMD).
In previous research supported by MDA, Worman's group showed that inhibiting proteins in the MAP kinase signaling pathway improved heart function in a mouse model of EDMD.
"The 'first-generation' inhibitors that we used are not suitable for humans," Worman said. "In the current research, we will study drugs of the same classes that have already been given to humans to determine if they similarly improve heart function in these mice."
The group plans to administer the new inhibitors and then examine the mice using some of the same methods doctors use to examine the heart in humans, including echocardiography (a type of imaging study used to assess the heart's pumping ability). The investigators will extend their findings to other EDMD mouse models known to develop cardiomyopathy, and then ascertain whether administration of MAP kinase signaling inhibitors causes toxicity in these mice.
Positive results generated by Worman's research could pave the way for clinical trials of MAP kinase signaling inhibitors to treat humans with EDMD.
"MDA has supported our research on Emery-Dreifuss muscular dystrophy for more than 10 years," Worman said. "This most recent grant will allow us to translate these past 10 years of basic laboratory research into a treatment for human patients with muscular dystrophy."
Funding for this MDA grant began August 1, 2010.
Grantee: EDMD — Howard Worman
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - DMD/BMD — Thomas Rando, M.D., Ph.D.

MDA has awarded a research grant totaling $602,087 over three years to Thomas Rando, a professor in the department of neurology and neurological sciences at Stanford (Calif.) University School of Medicine. The grant will help support Rando's studies to understand how scar-tissue formation (fibrosis) occurs in muscular dystrophies, especially Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD). DMD is caused by an absence of the dystrophin protein, and BMD is caused by dystrophin protein that's only partially functional.
In skeletal muscles affected by muscular dystrophy, fibrosis develops as muscle fibers degenerate and are replaced by connective tissue.
"The goals of my experiments are to understand why fibrosis occurs in the muscular dystrophies and to determine the biochemical mechanisms that lead to it," Rando said.
"We have preliminary data that suggest that a specific biochemical pathway known as the TGF-beta signaling pathway is activated in dystrophic muscle and affects muscle stem cells in a way that leads to the development of fibrosis."
Rando and colleagues will directly test whether blocking this pathway leads to a reduction of fibrosis in the muscles of dystrophin-deficient mice that develop a DMD-like disease.
"These studies have the potential to lead directly to new therapies that will reduce the amount of fibrosis in the muscles of boys with Duchenne muscular dystrophy," Rando said.
Funding for this MDA grant began August 1, 2011.
Grantee: DMD/BMD — Thomas Rando, M.D., Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - DMD/BMD — Zolt Arany

MDA awarded $352,188 to Zolt Arany, assistant professor in medicine at Beth Israel Deaconess Medical Center, part of Harvard Medical School in Boston, for research into the role of skeletal muscle metabolism in Duchenne muscular dystrophy (DMD).
"It has become increasingly clear that skeletal muscle metabolism plays a critical role both in the onset of, and resistance to, neuromuscular diseases like DMD," Arany said. "Blood vessels are a critical component of metabolism, because they bring oxygen and nutrients to metabolically active tissues."
Arany's team recently uncovered a metabolic pathway that strongly induces the formation of new blood vessels in muscle. Previous work by others already has established that the pathway protects against muscle damage in a model of DMD, but it's not understood how it accomplishes this.
The new work is designed to test the hypothesis, in the mdx mouse model of DMD, that the metabolic pathway protects against muscle degeneration and atrophy by boosting the density and activity of blood vessels.
"Without support from the MDA, we would not be able to initiate these important studies," Arany said. "MDA has had an important impact on research in muscle dystrophy, and its role now, in times of federal budgetary constraints, is more critical and influential than ever."
A greater understanding of precisely how this particular metabolic pathway protects skeletal muscle may lead to novel therapeutic approaches for DMD and other devastating muscle diseases.
Funding for this MDA grant began August 1, 2010.
Grantee: DMD/BMD — Zolt Arany
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2011 - DMD/BMD — Terence Partridge, Ph.D.

MDA has awarded a research grant totaling $474,278 over three years to Terence Partridge, a professor of pediatrics at George Washington University Medical School and Children's National Medical Center in Washington, D.C. The award will help support Partridge's work to improve the usefulness of the standard mouse model of Duchenne muscular dystrophy (DMD), the so-called "mdx" mouse.
The mdx mouse lacks dystrophin, the protein that's missing in people with DMD and only partially functional in people with the related disease Becker muscular dystrophy (BMD).
The muscular dystrophy developed by the mdx mouse "closely resembles DMD in some respects," Partridge said, "but differs in others. In order to make the best use of this valuable animal model, we need to fully understand the extent of the similarities and differences, so that we can take them into account when applying information gathered from the mouse to the situation in man."
Partridge and colleagues will develop reliable methods for making accurate and reproducible measurements of the rates of growth, death and regeneration of muscle taking place at different times in the mdx mouse.
"Nowadays, funding from MDA is especially important to those of us working on the interface between science and translational research," Partridge said. "MDA provides for the conduct of research that is fundamental in nature but is aimed at providing information that is directly relevant to the understanding of the disease processes or to the development of therapeutic agents to moderate those disease processes."
Funding for this MDA grant began August 1, 2011.
Grantee: DMD/BMD — Terence Partridge, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country:
Grant - Summer 2010 - DMD/BMD — Vihang Narkar
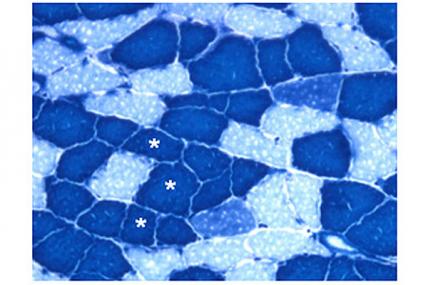
Vihang Narkar, assistant professor at the University of Texas Health Science Center at Houston, was awarded $302,326 to study the potential therapeutic value of increasing the overall amount of a specific type of muscle called "aerobic muscle" in Duchenne muscular dystrophy (DMD).
Loss of the structural protein dystrophin, the underlying cause of DMD, causes muscle instability, damage and progressive weakness, accompanied by a decrease in the ability to produce energy, along with severe fatigue.
Because aerobic muscles have enhanced capacity for energy production (which takes place inside cell compartments called mitochondria) and are resistant to fatigue, it's thought that biological regulators that could stimulate production of this type of muscle in individuals with DMD might ameliorate some of the symptoms in the disease.
Narkar's group has discovered one such "bio-regulator," a protein called ERRgamma. When activated in the skeletal muscles of mice with a DMD-like disease, ERRgamma prompts production of a highly aerobic and fatigue-resistant muscle.
In their new work, Narkar and colleagues plan to genetically activate ERRgamma in the skeletal muscles of mdx mice, a rodent model of DMD, as a means of increasing the overall amount of aerobic, fatigue-resistant muscles. A sophisticated battery of tests will help the investigators evaluate the effects of ERRgamma on muscle health and determine whether targeting the protein to dystrophic muscles may have therapeutic benefit in DMD.
"This is our first research grant from the Muscular Dystrophy Association," Narkar said. "It will play a very critical role in initiating and advancing this new and therapeutically promising area of research."
Funding for this MDA grant began August 1, 2010.
Grantee: DMD/BMD — Vihang Narkar
Grant type: Research Grant
Award total:
Institution:
Country:
MDA Resource Center: We’re Here For You
Our trained specialists are here to provide one-on-one support for every part of your journey. Send a message below or call us at 1-833-ASK-MDA1 (1-833-275-6321). If you live outside the U.S., we may be able to connect you to muscular dystrophy groups in your area, but MDA programs are only available in the U.S.