
A Muscle Disease Concealed by a Muscular Physique

Every grade school has at least one: that scrawny kid who's a laughingstock in school sports and a favorite punching bag for bullies.
In many ways, Christoph Lossin was that kid, except that he was anything but scrawny. His athletic attempts usually ended in embarrassment, and he was a sure loser in fistfights. But he was also a strapping young man, with bulging muscles in his arms and legs. Unfortunately, when he needed them most, those impressive-looking muscles always seemed to let him down.
As in the 50-yard dash — a race that's part of an annual fitness test for schoolchildren in the United States and abroad. "If I crouched down in the typical starting position, I would immediately freeze once I stood," recalls Lossin, who was born and raised in Germany. "My legs would lock into complete extension. It was like trying to run on stilts. I'd actually start running in maybe the last 20 or 30 meters."
That same stiffness could abruptly take hold all over Lossin's body, making him an easy target in a fight.
"I looked like a buff guy, and other kids thought, "let's try and rough him up a little bit.' But of course I couldn't defend myself," he says. "If some other kid shoved me ... just trying to right myself, I'd freeze in the middle of action and then fall over like a piece of wood."
At the time, many people thought Lossin's problems were psychological. Lossin's teachers thought he just had a bad attitude, doctors thought he was going through "growing pains," and Lossin himself had occasional doubts about his mental health. Why wouldn't his athletic-looking physique translate into athletic performance?
The answer came when Lossin was 14 and an astute doctor diagnosed his condition as myotonia congenita — not a psychological disorder, but a rare, inheritable disease of muscle.
A 'Misunderstood' Disease
The name sums up the main features of the disease: muscle stiffness (myotonia) that begins sometime in childhood, from as early as birth (congenital). The stiffness, sometimes referred to as cramping, is more accurately described as an inability to relax skeletal (or voluntary) muscles after contraction. It's usually chronic at some level, and can cause discomfort during simple motions like walking, grasping and chewing.
Worst of all, with sudden movement after a period of rest, it can become debilitating, even paralyzing.
During sustained movement, the stiffness usually fades away, causing what's called the "warm-up" phenomenon. In other words, warming up, or preparing for physical activity with low-intensity movements — for example, repeatedly making a fist before grasping and lifting a heavy object — can largely prevent severe attacks of myotonia. Unfortunately, many situations — for example, tripping or getting shoved by a passerby — can trigger sudden, reflexive movements without an opportunity for warming up.
Another hallmark of myotonia congenita is the muscle enlargement (hypertrophy) that made Lossin's problems look as if they weren't in his body, but in his head.
"It's a disease that's very easily misunderstood," Lossin says. "When people look at me, they see somebody who looks perfectly normal, for one thing, because I know how to hide [the myotonia] and also because I'm usually warmed up. But if I had to suddenly jump up and run out of a building during a fire, they would see it."
As a child, of course, Lossin himself didn't understand the nature of his disease, but as an adult he's able to describe it down to its molecular details. Lossin, now 28, is studying toward a Ph.D. in neuroscience at Vanderbilt University in Nashville, Tenn., where he's working in a research lab run by one of the country's leading experts on myotonia congenita. He ended up there because he was once driven to know everything he could about his obscure disease.
Chloride Channels
Although early theories speculated that myotonia congenita was rooted in the nervous system (the brain, spinal cord or nerves that control muscles), it's now known to originate from genetic defects in skeletal muscle itself.
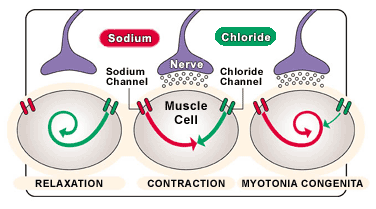
Normally, when a nerve cell stimulates a muscle cell, it triggers a flow of ions — charged particles of sodium, potassium, calcium and chloride — across the outer surface (membrane) of the muscle cell. That flow of ions creates a short-lived electrical current, which ultimately causes muscle contraction. The ions flow into and out of muscle cells through ion channels — tiny protein-based pores in cell membranes that open to let specific ions pass, and close to shut them out.
Myotonia congenita is caused by mutations in the CLCN1 gene on chromosome 7, which encodes the skeletal muscle chloride channel. When this channel is open, it normally works against the electrical current that causes muscle contraction (see illustration). In most cases of myotonia congenita, the chloride channel doesn't open enough.
Consequently, "the muscle will generate too many electrical impulses and the contraction will persist," says neurologist Alfred George, Lossin's mentor at Vanderbilt and a former MDA grantee. Eventually, other mechanisms appear to kick in and bring the muscle back to its normal cycles of rest and contraction, George says.
That explains why myotonia congenita intensifies with sudden movements and wanes with sustained movement. And there's a logical explanation for why the disease tends to cause muscle hypertrophy as well.
"The myotonia is creating a higher level of resistance, and movement against resistance is a stimulus for muscle growth," George says.
What scientists don't understand is why the disease exists in two distinct forms. Becker-type myotonia congenita, sometimes called generalized myotonia, is the more common version of the disease and the form Lossin has. It's inherited in an autosomal recessive manner, meaning that it requires two copies of the defective CLCN1 gene.
The second form, Thomsen's disease (see "Thomsen's Legacy,"), is relatively rare, and is inherited in an autosomal dominant manner, meaning that it requires only one copy of the defective CLCN1 gene. Strangely, the two forms of the disease can be caused by identical mutations in CLCN1.
A Tale of Two Ions
Normally, in a relaxed muscle cell, chloride channels in the cell membrane are open, allowing a current of negatively charged chloride ions to pass into the cell. This creates a negative membrane potential — meaning there's an accumulation of negative charge on the inside of the membrane relative to the outside.
A nerve cell triggers contraction of the muscle cell by turning the membrane potential from negative to positive. Chemicals from the nerve cell stimulate the opening of sodium channels in the muscle cell, causing an inward current of positively charged sodium ions. When the positive current overcomes the negative (chloride) current, the muscle cell contracts.
In muscle cells affected by myotonia congenita, defective chloride channels reduce the entry of chloride into the muscle cell or allow excess sodium to enter. Therefore, just a small amount of sodium influx is enough to change the membrane potential and cause contraction. The contraction is sustained by a buildup of positively charged ions at the muscle cell membrane, and relaxation is delayed.
Dangerous, But Not Always Disabling
The level of disability caused by myotonia congenita varies depending on the disease form, and on the affected individual, says neurologist Louis Ptacek, an MDA grantee at the University of Utah in Salt Lake City. Becker-type tends to be more severe than Thomsen's disease, and in some people, it causes muscle weakness and wasting in addition to myotonia, he says.
"There are some Becker patients who end up in wheelchairs. Then, there are others who lead relatively normal lives, remain ambulatory, and are able to control their myotonia simply by modifying their lifestyles," Ptacek says. "I have a patient with Thomsen's disease who's actually a heavy equipment operator, and he does quite well."
Attacks of myotonia cause a "sense of tightness," Ptacek says, but aren't usually painful. There are exceptions to that rule, George says. "The textbook description of myotonia congenita is painless muscle stiffness, but that's definitely not the universal experience. A number of patients describe muscle cramping or an aching feeling, especially the active people who exercise or compete in sports."
For reasons that aren't clear, stress and anxiety seem to aggravate symptoms — a trend that once reinforced the idea that myotonia congenita was a neurological disorder.
But experts say the most striking feature of the disease is that it can turn a simple reflexive action into a dangerous accident.
"You might read that myotonia congenita isn't life-shortening," Lossin says, "but I recall many times that I've tripped and, simply because I couldn't right myself, I fell over like a board and hit my head very hard. I was sometimes amazed that I didn't get hurt worse. That's probably the most severe thing about the disease — the danger of getting into a situation that can cost you your life because you can't react smoothly with your muscles."
"I've met patients who've had significant injuries when they've fallen," George confirms. "I've also heard reports of people who are suddenly distracted while driving, and they turn sharply toward the distraction and have trouble turning toward the front of the car again. Fortunately, I've never heard of anyone actually getting into an accident that way."
Discoveries
For Lossin, the physical limitations of myotonia congenita have been small compared to the less tangible injuries, measured in stares, taunts and other reactions from ignorant people over the years. Those responses, and sometimes just the perception of them, significantly shaped Lossin's life, from everyday behaviors to career decisions.

"There were so many things that I did or did not do because of myotonia congenita," he says. "It directed my life for a long time. I wanted to get into science because of it."
When Lossin received his diagnosis in the 1980s, research on myotonia congenita was in its early stages. About a decade earlier, pharmacologist Shirley H. Bryant had begun pioneering experiments to discern the cause of the disease.
Bryant had come across a report about a herd of goats with myotonia congenita, discovered because they habitually grazed next to a train track. (Every time a train passed by, the goats tried to run, but then froze and collapsed.) While at the University of Cincinnati in Ohio, he received an MDA grant to study the myotonic goats, and found that their muscles had a decreased capacity to take up chloride.
Building on Bryant's work, Thomas Jentsch of Hamburg University in Germany identified a strain of myotonic mice and found that the mice had a mutation in CLCN1. That prompted Jentsch to search for CLCN1 mutations in people with myotonia congenita, and in an MDA-funded study published in 1992, he found evidence for several CLCN1 mutations in different families.
Soon after, Ptacek and George published studies showing that most CLCN1 mutations interfere with the opening of the muscle chloride channel.
Those discoveries fueled Lossin's desire to go into myotonia congenita research. And, perhaps most importantly, they created an opportunity for him and others to get diagnoses based on genetic testing.
"When I had the test done in 1997, I was a graduate student, so I was reading the scientific papers [about myotonia congenita]. When I got the results ... all of a sudden, I could explain to people what was causing my myotonia. It was an elating feeling," he says. "For people who suffer from these rare conditions, I think it's important to have the assurance that you're not imagining this and you're not making it up."
But not everyone is lucky enough to get confirmation from a genetic test. Because myotonia congenita is rare and the underlying CLCN1 mutations are diverse, testing for the disease is limited. In the United States, the only facility that currently offers such tests is the Center for Research in Genetic Medicine, run by MDA grantee Eric Hoffman at Children's National Medical Center in Washington.
For most people, "the diagnosis of myotonia congenita is a clinical diagnosis [based on symptoms] with supportive evidence from the EMG [electromyogram] and family history," George says. Another important part of diagnosis is exclusion of the more common disease myotonic muscular dystrophy. In most people, this disease is caused by a genetic defect on chromosome 19 that's detectable by a commercially available test.
Thomsen's Legacy
"When I was a kid, I used to ask myself "Why don't my muscles do what I want them to do?'" recalls Christoph Lossin, who was found to have myotonia congenita during his childhood in Germany.
One hundred years earlier, the same question troubled German physician Asmus Julius Thomsen, who became the first person to describe myotonia congenita — in himself and his family. Thomsen grew up frustrated and embarrassed by his attacks of myotonia congenita, and as an adult, he apparently did his best to conceal them from his medical colleagues.
But when Thomsen was 61, one of his sons, also affected by myotonia congenita, was accused of malingering his way out of military service. Rallying to his son's defense, Thomsen finally revealed his secret and described his family's condition in a German medical journal published in 1876.
Like young Lossin, Thomsen fixated on the observation that his mind couldn't seem to take command over his muscles. This line of thinking, combined with the unfortunate coincidence that mental illness also ran through his family, led Thomsen to propose that myotonia congenita originated not in muscles, but in the brain or nerves.
Although that theory turned out to be wrong, Thomsen's detailed account of myotonia congenita in his own family captured the now-classic features of the disease and even pointed to its hereditary nature. Since Thomsen's family had autosomal dominant myotonia congenita, this form of the disease became known as Thomsen's disease.
Managing Myotonia
For Lossin and many other people, a firm diagnosis of myotonia congenita not only has relieved anxiety — it's led to effective ways of coping with the disease.
"There are a number of different medications available, and many patients take some type of medication on a daily basis," Ptacek says. "But drugs that work well for one patient might not be tolerated by another. The best thing to do is slowly start up a new medication to an appropriate dose, and see if there's some benefit." (For more on drugs, see "Treatments, Today and Tomorrow.")
For Lossin, none of the available medications was helpful. Instead, he's found relief simply by staying in shape and staying "warmed up." In the process, he's become the athlete he never thought he could be.
He works out two to three times per week, with a regular routine that includes exercises like running on a treadmill or jumping rope. He even plays competitive badminton and probably has the best "smash" move at Vanderbilt, he says.
"After I work out I become extremely myotonic," he admits. "I come home and my wife has to undress me or I get painful cramps. But overall, I feel better maintaining a certain level of physical fitness than not being in shape at all."
But warming up helps him keep the cramps under control. "Myotonia doesn't prevent you from doing anything as long as you're warmed up and prepared," he adds. He has to warm up before intense activities like working out, and even before simple, everyday activities like climbing stairs.
But for Lossin, perhaps the real key to managing myotonia congenita has been overcoming ignorance about it. "Nowadays, most of my close friends know about my condition," he says. That means they know not to horse around in ways that could seriously injure him, like jostling him with a friendly punch or giving him a surprise shove into a pool. "They also know that if I trip and suddenly grab them, it's because I would be on the ground if I didn't."
With his once-insatiable quest for knowledge about myotonia congenita, Lossin has helped himself and others understand the disease. He's even given demonstrations of his myotonic attacks in medical school classes taught by George.
He's learned to live with myotonia congenita, mostly by conquering the frustration and embarrassment that it used to cause him. Achieving that peace of mind — and finding love — have ultimately made his quest seem less urgent, he says.
"Once I met my wife, my incentive of being a great myotonia researcher lost its drive, because she accepts me the way I am," he says. For now, he's content to study other projects in George's lab.
Treatments, today and tomorrow

For some people, myotonia congenita causes mild symptoms that can be managed through lifestyle modifications. But others experience significant physical disabilities that require medical treatment. There are several effective drug treatments for myotonia congenita, and with research, better treatments may be on the way.
All of the available drugs for myotonia congenita act on ion channels, but none of them actually target CLCN1, the ion channel that's defective in the disease. Instead, most of them work by temporarily plugging up sodium channels.
"It's easier to shut down an ion channel than it is to rev up an ion channel," neurologist Louis Ptacek explains. "When the chloride channels in muscle aren't working well enough, a muscle becomes hyperexcitable because of sodium channels opening. So, a drug that blocks the sodium channels when they're open will counteract the myotonia." (See "A Tale of Two Ions," for more about the role of sodium channels in myotonia.)
To understand how the drugs work, it's also important to know that ion channels control vital processes in many organs and tissues besides muscle. For instance, they regulate the firing of nerve cells in the brain, the rhythmic beating of the heart and the selective excretion of waste products by the kidneys. Each tissue has its own distinct complement of ion channels, encoded by different genes. (The CLCN1 gene encodes a chloride channel that's exclusively found in skeletal muscle.)
Despite those tissue-specific differences, the ion channels in muscle work much the same as channels in other tissues. So, several ion-channel-blocking drugs originally developed for other purposes — including anti-convulsants (traditionally used for preventing seizures in the brain), diuretics (which increase urine output by the kidneys) and anti-arrhythmics (which protect against cardiac arrhythmia) — also work well for myotonia congenita.
Many physicians say the most effective drug for myotonia congenita is the anti-arrhythmic mexiletine (Mexitil). When used at an appropriate dose, mexiletine can alleviate myotonia without affecting the heart. The most common side effects are tingling around the mouth, light-headedness, dizziness and nausea, neurologist Alfred George says. Unfortunately, he adds, "many patients say mexiletine works great for a while but the side effects become too much, or it just becomes ineffective."
With an eye toward better treatments, Ptacek is continuing to investigate the CLCN1 mutations that cause myotonia congenita.
"The most immediate benefit of these studies will be an improvement in our ability to diagnose patients by genetic testing," he says. "The long-term benefit is that for clinical trials of new drugs, we'll be able to select patients who have myotonia congenita that's been verified by a genetic test." If a drug trial were to include patients with a mixture of diseases similar to myotonia congenita, any benefits from the drug would be difficult to see, he explains.
Meanwhile, in an effort supported by MDA, George is developing a new gene therapy approach that might be useful for treating myotonia congenita. Instead of targeting the chloride channel gene, the approach is designed to repair the chloride channel's RNA — the chemical string of letters that normally serves as an intermediate between DNA (genes) and proteins. "The idea is that if you could change the mutated sequence in the RNA back to the normal sequence, then a normal protein would get made," George says.
The technique, which makes use of ribozymes (unusual bits of RNA that never get made into protein, but instead cut and paste other RNAs), has worked in isolated cells with myotonia congenita mutations, George reports. Next, he plans to test it in dogs that have myotonia congenita, perhaps by using a virus to deliver the ribozymes to specific muscles.
Unfortunately, unless viral delivery techniques improve, getting the ribozymes to muscles throughout the body is likely to require surgery, George says. Thus, the treatment might turn out to be too invasive for most people with myotonia congenita, but might be appropriate for people with more severe diseases like Duchenne muscular dystrophy.
MDA Resource Center: We’re Here For You
Our trained specialists are here to provide one-on-one support for every part of your journey. Send a message below or call us at 1-833-ASK-MDA1 (1-833-275-6321). If you live outside the U.S., we may be able to connect you to muscular dystrophy groups in your area, but MDA programs are only available in the U.S.
Request Information