Duchenne Muscular Dystrophy (DMD)
Causes/Inheritance
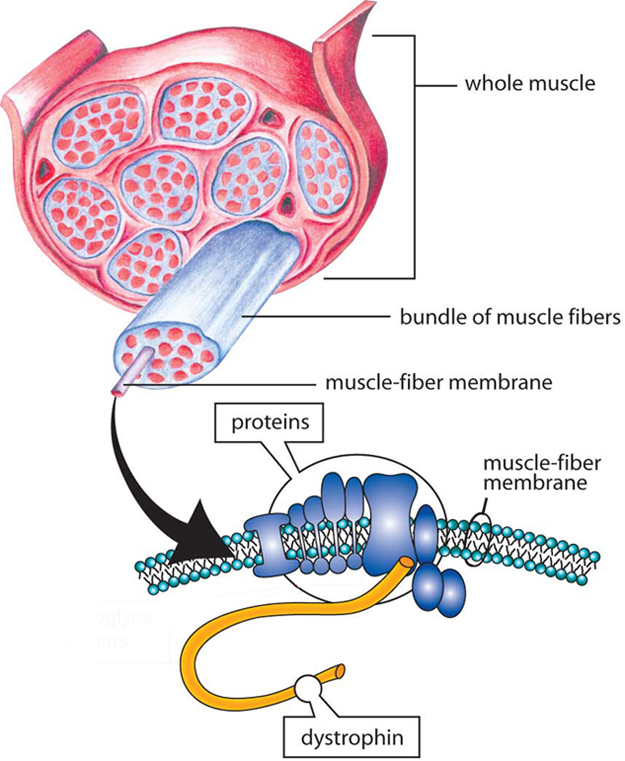 Cause of DMD
Cause of DMD
Until the 1980s, little was known about the cause of any of the forms of muscular dystrophy. In 1986, MDA-supported researchers identified a gene on the X chromosome that, when flawed (mutated), causes Duchenne, Becker, and intermediate forms of muscular dystrophy.
Genes contain codes, or recipes, for proteins, which are important biological components in all forms of life. In 1987, the protein associated with the DMD gene was identified and named dystrophin. The DMD gene is the largest gene in humans and is located in the short arm of the X chromosome, in the Xp21.2 locus (a locus is the position of a gene on a chromosome). The majority of mutations of the DMD gene are deletions of one or more parts of it.
DMD occurs because the mutated DMD gene fails to produce adequate amounts of functional dystrophin. Individuals with BMD genetic mutations make partially functional dystrophin, which somewhat protects their muscles from degenerating as severely or as quickly as in DMD.
During a muscle cell contraction, the dystrophin protein transfers the force of muscle contraction from the inside of the muscle cell outward to the cell membrane. Because it connects the center of the muscle cell to the edge of the cell, the dystrophin protein is extremely long. One end is specialized for linking to the muscle cell interior and the other end is specialized for linking to a variety of proteins at the cell membrane. The long middle section, called the rod domain, is taken up by a series of repeating units called spectrin-like repeats. Many cases of DMD are caused by mutations in the part of the gene that encodes this middle section. Production of the entire protein stops when the mutation is encountered.
The absence of dystrophin sets in motion a cascade of harmful effects. Without dystrophin, muscles are more susceptible to injury during contraction, which triggers inflammation. Over time, chronic inflammation causes fibrous and fatty tissue to replace healthy muscle, severely compromising muscle function. Beyond its role in force-transfer, dystrophin also acts as a scaffold that anchors numerous molecules in place near the cell membrane. Loss of dystrophin disrupts the positioning of these molecules, which consequently disrupts their functions. An example of a functional disruption that occurs in DMD is the decrease of blood flow to muscles. Ultimately, lack of dystrophin causes muscle damage and progressive weakness, beginning in early childhood.
Inheritance in DMD
DMD is inherited in an X-linked pattern because the gene that can carry a DMD-causing mutation is on the X chromosome. Every male has an X chromosome from his mother and a Y chromosome from his father. Females get two X chromosomes, one from each parent.
Each son born to a female with a DMD gene mutation on one of her two X chromosomes has a 50% chance of inheriting the flawed gene and having DMD. Each of her daughters has a 50% chance of inheriting the mutation and being a carrier. Carriers may have minimal to no symptoms, but can have a child with the mutation or the disease with subtler symptoms of weakness, cramps, and heart problems (cardiomyopathy).
Although DMD often runs in a family, it is possible for a family with no history of DMD to suddenly have a son with the disease. There are two possible explanations. The first is that the genetic mutation leading to DMD may have existed in the females of a family for some generations without anyone knowing. Perhaps no male children were born with the disease, or, even if a boy in an earlier generation was affected, relatives may not have known what disease he had.
The second possibility is that a child with DMD has a new genetic mutation that spontaneously arose in one of his mother’s egg cells. Because this mutation is not in the mother’s blood cells, it is impossible to detect by standard carrier testing. With the growing use of prenatal genetic testing and adoption of newborn screening for DMD, the incidence of spontaneous (also called de novo) cases of DMD is increasing, representing nearly one third of all new DMD cases. Conversely, the number of familial cases of DMD is decreasing due to genetic counseling and family planning.
A man with DMD cannot pass the flawed gene to his sons because he contributes a Y chromosome, not an X chromosome, to them. However, all of his daughters will be obligate carriers, since each daughter inherits his only X chromosome, which carries the DMD mutation. As carriers, these daughters have a 50% chance of passing the mutated gene to each of their children. This means that each of the man’s grandsons has a 50% chance of inheriting DMD, and the pattern can continue in subsequent generations.
Females and DMD
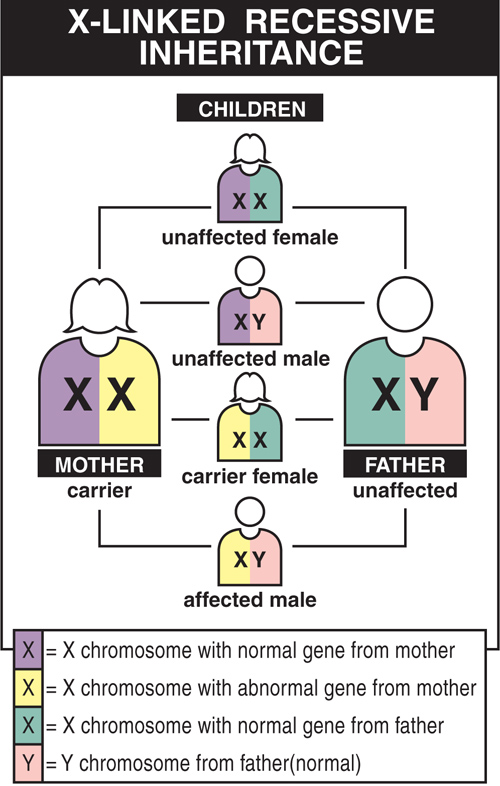
Why don’t girls usually get DMD?
When a female inherits a flawed DMD gene from one parent, she usually also gets a healthy DMD gene from her other parent, which gives her enough healthy protein to protect her from the most serious symptoms of the disease. Males who inherit the mutation get the disease because they have no “back-up” second DMD gene to make up for the faulty one.
Early in the embryonic development of a female, either the X chromosome from the mother (maternal X) or the one from the father (paternal X) is inactivated in each cell. Chromosomes become inactivated at random. In each cell, there is a 50% chance that either the maternal or paternal X chromosome will be inactivated, with the other left active. In rare cases, females may show signs of DMD if their cells preferentially use the copy of the X chromosome that carries the DMD mutation – a situation called “skewed X-inactivation.”
Usually, females do not experience the full disease symptoms of DMD as males do, although they can still have symptoms of muscle weakness. A minority of females with DMD mutations, called manifesting carriers, have some signs and symptoms of DMD. For these females, the dystrophin deficiency may result in weaker muscles that fatigue and cramp more easily. Manifesting carriers may have heart problems, which can show up as shortness of breath or an inability to do moderate exercise. The heart problems, if untreated, can be quite serious, even life-threatening, requiring monitoring by a cardiologist.
In very rare instances, a female may lack a second X chromosome entirely, or her second X chromosome may have sustained serious damage. In these cases, she makes little or no dystrophin (depending on the type of dystrophin mutation), and develops a dystrophinopathy just as a male would.
A female relative of a boy with DMD can get a full range of diagnostic tests (i.e., genetic counseling) to determine her carrier status. This can help her understand the risks and plan for medical care. If she is found to be a DMD carrier, regular strength evaluations and close cardiac monitoring can help her manage any symptoms that may arise.
Additional reading
- Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell (1987). doi:10.1016/0092-8674(87)90579-4
- Venugopal V, Pavlakis S. Duchenne Muscular Dystrophy. [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482346/
Last reviewed May 2025 by Stephen Chrzanowski M.D., Ph.D.

