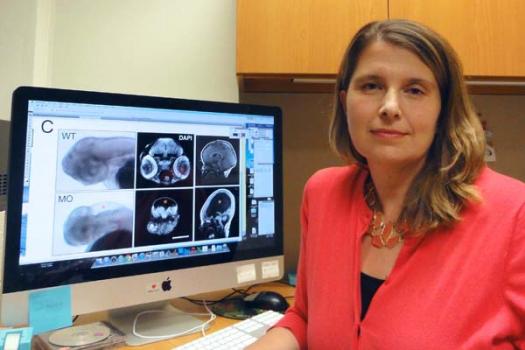
Carmen Bertoni, assistant professor of neurology at the University of California, Los Angeles, was awarded an MDA research grant totaling $300,000 over a period of three years to advance gene-editing strategies forDuchenne muscular dystrophy (DMD).
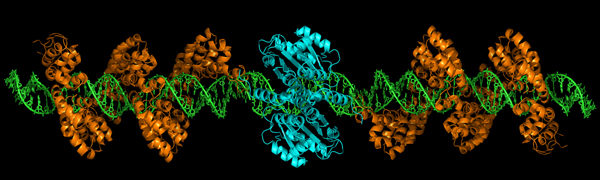
DMD is caused by a mutation in the dystrophin gene. Standard gene therapy techniques have focused on supplying an unmutated dystrophin gene to muscle cells. An alternative strategy, called gene editing, seeks to correct the mutant gene itself.
“Our research group [with MDA funding] has pioneered the use of gene editing strategies for the dystrophin gene to permanently correct the DNA,” Bertoni says.
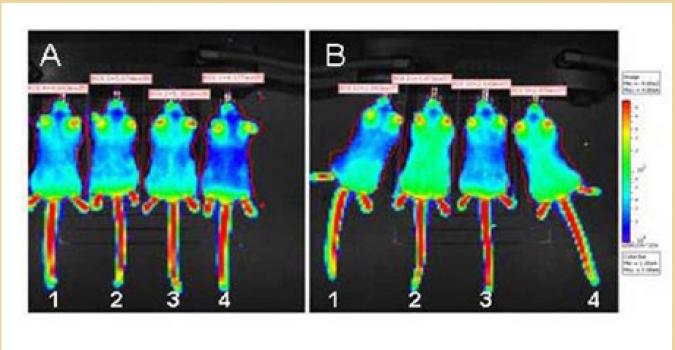
The gene-editing toolkit is growing, and in this project, Bertoni will compare the correction efficiency of two different methods. Both specifically target the mutant sequence in the gene, and harness elements of the cell’s own “quality control” system to correct the mutation. The comparison will be performed first using muscle cells isolated from a mouse model for DMD, and then in the mice themselves, to determine the feasibility of using this technology in people.
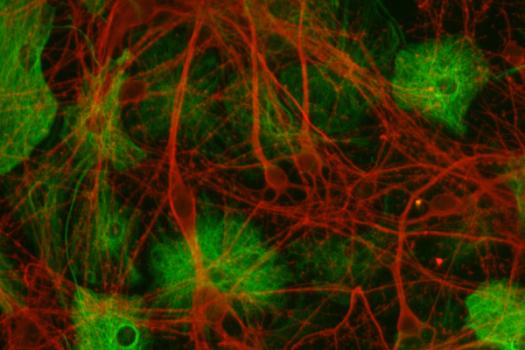
The repair process is simple to describe, but complicated in practice. The therapy needs to reach many different muscle fibers, cross the dense and protective structure that surrounds every fiber, reach the cell nuclei and ultimately the dystrophin gene, and then direct the gene correction process. Finally, the amount of dystrophin expression achieved after correction needs to be sufficient to sustain muscle function over a prolonged period of time.
“Every one of these steps is necessary to ensure a safe and effective therapy in patients,” says Bertoni. “Altogether these studies have the potential to significantly advance the field of gene correction into a clinical scenario for the treatment of DMD.”
Funding for this MDA grant began August 1, 2013.
Grantee: DMD — Carmen Bertoni, Ph.D.
Grant type: Research Grant
Award total:
Institution:
Country: